Hardness: 6.5 - 7.
D = 2.68 to 3.03 gm./cm3.
Luster: vitreous, greasy to dull. Streak: white.
Isotropic or very weakly anisotropic, n 1.507 - 1.525
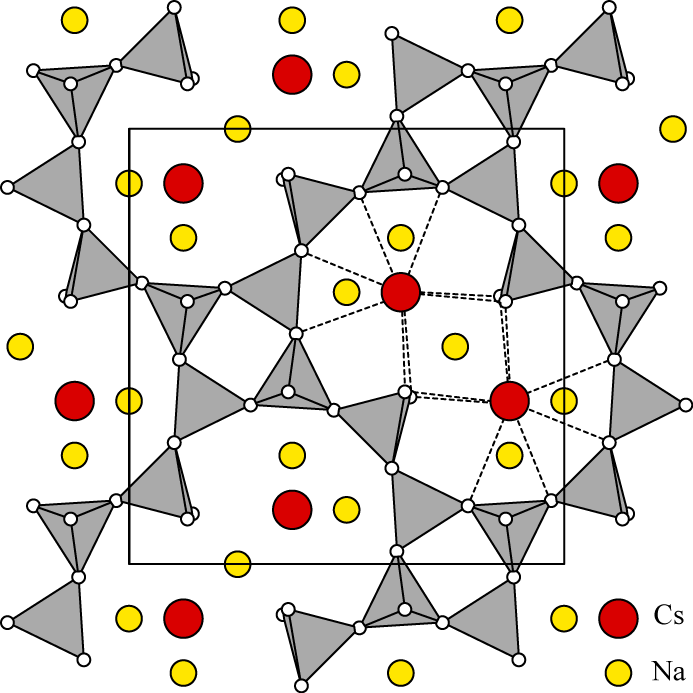
Teertstra et al. (1994) studied the Si,Al disorder of cesian analcime and pollucite with magic-angle-spinning nuclear magnetic resonance spectroscopy. Their results show that isotropic pollucite is largely disordered, while anisotropic samples have a slight degree of ordering.
With modern instrumental analyses small amounts of several non-framework cations, other than Cs and Na, can be determined. Rb and K are commonly present, and Li, Ca, and Mg, in lesser amounts. The composition of pollucite samples are expressed in two slightly different ways. %CRK (Cs+Rb+K) represents the total cation occupancy in the W site, while %Poll is only the Cs content. CRK cations -- Na‑site cations -- Si variations for some examples of pollucite from Teertstra and Černý (1995) and Teertstra et al (1992) are shown in the accompanying figure.
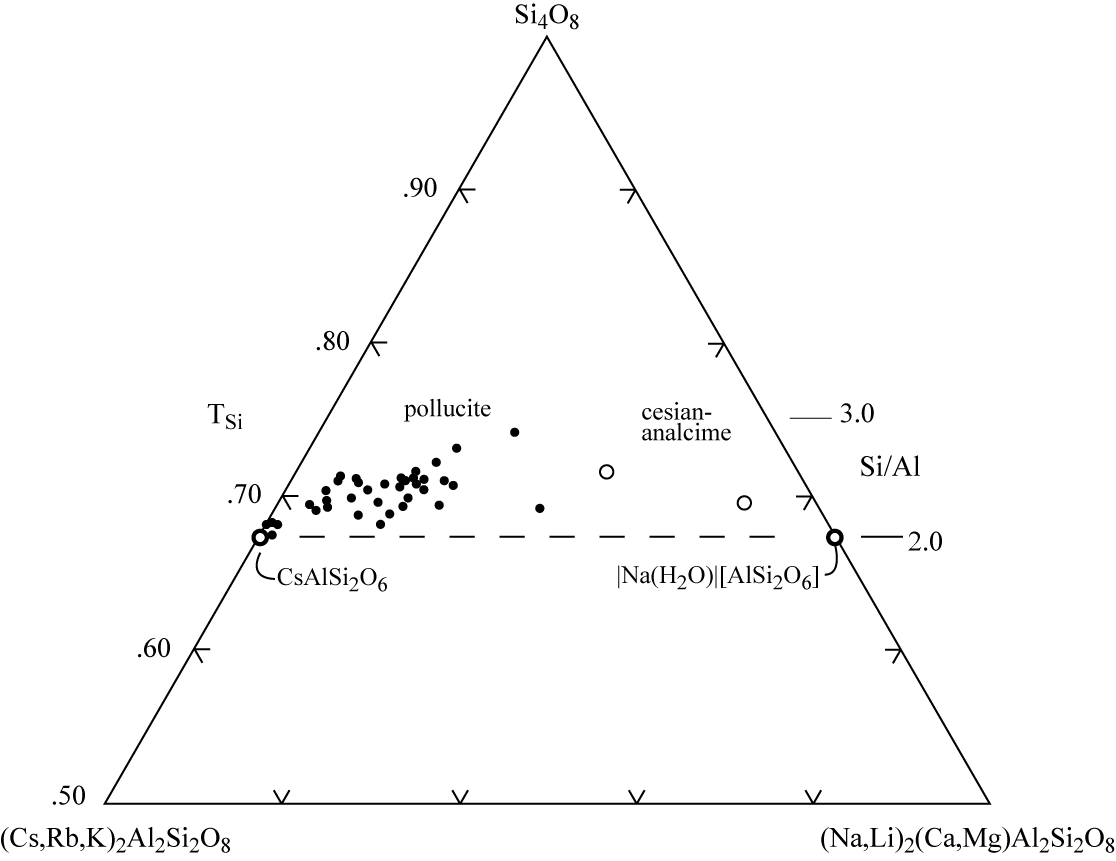
Primary pollucite samples contain 70 to 80% CRK, while recrystallized pollucite compositions are somewhat more sodic or much more cesian, tending toward the pure pollucite end member (Teertstra and Černý 1995). The cesian analcime samples are from veins of low temperature alteration products in spodumene of the Tanco pegmatite at Bernic Lake, Manitoba, Canada (Černý 1972).
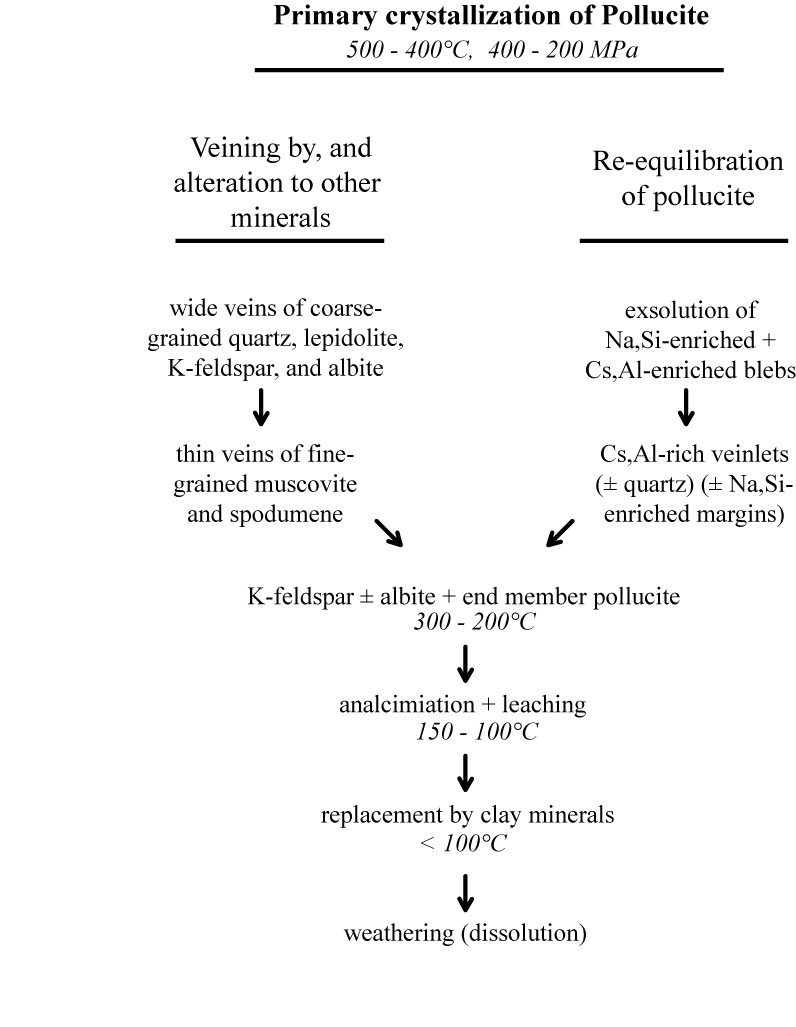
Pollucite has been interpreted as occurring mainly in two ways (Černý 1982), primary crystallization and late metasomatic alteration. Dymkov (1953), Quensel (1956), Hutchinson (1959), Ginsburg (1960 and others), Solodov (1960), and Wright (1963) argue for a late metasomatic origin. Textural evidence comes mainly from dikes with low pollucite content, in which granular, dispersed pollucite is associated with Ta, Nb, Sn, B. and P minerals of the typical albitization assemblages. Beus (1960), Melentyev (1961, 1970) Symons (1961), Cooper (1964), Crouse and Černý (1972), Heinrich (1976), Černý and Simpson (1978), Černý (1982), Teertstra et al. (1992), and Teertstra and Černý (1995) consider pollucite as a primary constituent of the near-central parts of pegmatite dikes. Evidence supporting this view comes from those dikes, in which pollucite forms large pods or lenses in well defined bodies. Boundaries of these pods are simple, showing no evidence of cross-cutting or replacement with other minerals or zones of minerals. Černý (1982) comments that the evidence for a metasomatic origin resembles the relationships seen in secondary crystallization and alteration in zones surrounding primary pods.
Teertstra and Černý (1995) estimate that primary pollucite crystallizes from a water-rich melt late in the solidification of the dike, probably in the temperature range 550 to 400°C. Based on minerals in fluid-solid inclusions from the Tanco pegmatite, London (1986) inferred the transition from magmatic to subsolidus hydrothermal conditions occurred between about 450° and 475°C. Where primary pollucite crystallizes in the presence of quartz, the composition is buffered by quartz and albite giving a composition near %Poll = 80.
Beus, A.A. 1960. Geochemistry of beryllium and the genetic types of beryllium deposits. Akad. Nauk, SSSR, 329 pp. (English translation, Freeman and Co., 1966).
Breithaupt, A. 1846. Pollux. (Poggendorff’s) Annalen der Physik und Chemie 69, 439.
Černý, P. 1972. The Tanco pegmatite at Bernic Lake, Manitoba. VIII. Secondary minerals from the spodumene-rich zones. Can. Miner. 11, 714-726.
Černý, P. 1974. The present status of the analcime-pollucite series. Can. Mineral. 12, 334-341.
Černý, P. 1982. Mineralogy of rubidium and cesium. In, Černý, P. (ed.) Short Course in Granite Pegmatites in Science and Industry, Min. Assoc. Canada, Handbook 8, 149-161.
Černý, P. and Simpson, F.M. 1978. The Tanco pegmatite at Bernic Lake, Manitoba. X. Pollucite. Can. Mineral. 16, 325-333.
Cooper, D.G. 1964. The geology of the Bikita pegmatites. In, The Geology of Some Ore Deposits in Southern Africa II, 441-462.
Crouse, R.A. and Černý, P. 1972. The Tanco pegmatite at Bernic Lake, Manitoba. I. Geology and paragenesis. Can. Mineral. 11, 591-608.
Dana, J.D. 1868. A System of Mineralogy (5th ed.) John Wiley and Sons, New York, N.Y.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004). Rock Forming Minerals. vol. 4B.Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites. The Geological Society, London.
Dymkov, Yu. M. 1953. Morphology of pollucite aggregates and their genesis. Trudy Mineral. Mus. Akad. Nauk SSSR 5, 132-145.
Ginsburg, A.I. 1960. Specific geochemical features of the pegmatitic process. Rept. 21st Int. Geol. Congr. 17, 111-121.
Heinrich, E.W. 1976. A comparison of three major lithium pegmatites: Varuträsk, Bikita, and Bernic Lake. U.S.Geol.Surv., Prof. Paper 1005, 50-54.
Hutchinson, R.W. 1959. Geology of the Montgary pegmatite. Econ. Geol. 54, 1525-1542.
Lagache, M. 1995. New experimental data on the stability of the pollucite-analcime series: application to natural assemblages. Eur. J. Mineral. 7, 319-323.
London, D.1986. The magmatic--hydrothermal transition in the Tanco rare-element pegmatite: evidence from fluid inclusions and phase equilibrium experiments. Am. Mineral. 71, 376-395.
Melentyev, G.B. 1961. New find of pollucite in the granitic pegmatites of Sayan Mts. Dokl. Akad. Nauk SSSR 141, 950-953.
Melentyev, G.B. 1970. First find of pollucite-bearing pegmatites in Central Asia and new data on the conditions of cesium accumulation. Dokl. Akad. Nauk SSSR 192, 180-183.
Náray-Szabó, St. V. 1938. Die Struktur des Pollucits CsAlSi2O6.xH2O. Z. Kristallogr. 99, 277-282.
Quensel, P. 1956. The paragenesis of the Varuträsk pegmatite. Ark. Mineral. Geol. 2, 9-125.
Solodov, N.A. 1960. Distribution of alkali elements and beryllium in minerals of one of the zoned pegmatites of the Mongolian Altai. Geokhimiya 1960, 726-735.
Strunz, H. 1936. Die chemische Zusammensetzung von Pollucit. Z. Kristallogr. 95, 1-.
Symons, R. 1961. Operation at Bikita Minerals (Private), Ltd., Southern Rhodesia. Bull. Inst. Mining and Metall. 661, 129-172.
Treetstra, D.K., Lahti, S.I., Alviola, R. and Černý, P. 1992. Compositional heterogeneity of pollucite from High Grade Dyke; Maskwa Lake, southeastern Manitoba. Can. Mineral. 30, 687-697.
Treetstra, D.K., Lahti, S.I., Alviola, R. and Černý, P. 1993. Pollucite and its alteration in Finnish pegmatites. Geol. Soc. Finland, Bull. 368, 39 pp.
Treetstra, D.K., Sherriff, B.L., Zhi Xu, and Černý, P. 1994. MAS and DOR NMR study of Al-Si order in the analcime-pollucite series. Can. Miner. 32, 69-80.
Treetstra, D.K. and Černý, P. 1995. First natural occurrences of end-member pollucite: a product of low-temperature reequilibrium. Eur. J. Mineral. 7, 1137-1148.
Wright, G.M. 1963. Geology and origin of the pollucite-bearing Montgary pegmatite, Manitoba. Geol. Soc. Am. Bull. 74, 919-946.