Stilbite-Na |(Na,Ca0.5, K)9(H2O)26|[Al9 Si27 O72]

Hardness: 4 – 4.5
D: 2.14 – 2.21 gm/cm3.
Luster: vitreous, pearly on {010}.
Streak: white.
Biaxial ( -)
2Vx 30 - 50° Y = b, X ˄ a 5°
Dispersion: r < v, moderate
2Vx 45°, Y = b, X ˄ c 9° Dispersion: r < v, moderate
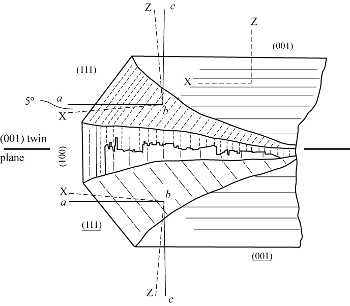
The framework type (STI) of the stilbite group, which includes stilbite series, stellerite, and barrerite, consists of two sets of connected channels. One channel extends parallel to the a-axis and is confined by a ten-membered ring (aperture 4.9 x 6.1 Å). The other channel (aperture 2.7 x 5.6 Å) is located along [101] for monoclinic frameworks or [001] for orthorhombic structure and is confined by an eight-membered ring. Both of these channels are in the (010) plane, creating a structural weakness across the plane leading to perfect (010) cleavage and a tabular habit.
Two different orientations have long been used to describe the crystallography of stilbite, the proper monoclinic setting with the β angle near 127° and a pseudo-orthorhombic setting with β near 91°. The second is commonly used for ease of comparison with crystal forms and crystal structures of stellerite and barrerite. Optical studies of stilbite have shown that within one macroscopic single-crystal, monoclinic (F2/m) and orthorhombic (Fmmm) coexist depending on the growth direction (Akizuki and Konno 1985). See diagram above. Akizuki et al. (1993) also studied the symmetry of different growth sectors in stilbite by single-crystal diffraction and refined the structure of an orthorhombic {001} growth sector yielding space group Fmmm, a = 13.616, b = 18238, c = 17.835 Å within a chemically homogeneous crystal. This structure represents a disordered variant of the monoclinic F2/m structure of stilbite where higher symmetry occurs on a submicroscopic scale rather than microscopically as in {101} growth sectors. Galli (1971) and Akizuki et al (1993) implied complete (Si,Al) disorder based upon average T-O distances. Slaughter (1970) and Quartieri and Vezzalini (1987) assumed pronounced (Si,Al) ordering, with Al contents varying between 11 and 40% in the different tetrahedral sites.
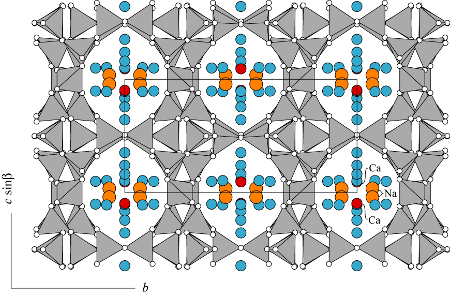
All refinements done in monoclinic symmetry yielded one fully occupied Ca (red) site located near the middle of the ten-membered ring parallel to the a-axis (see the figure). Ca is bonded only to channel H2O molecules and not to framework oxygens. Galli (1971) and Quartieri and Vezzalini (1987) found one partially occupied Na site (orange) that is seven-coordinated to two framework oxygens and five channel H2O molecules. Slaughter (1970) interpreted the Ca site to contain a small amount of Na and found three partially occupied Na sites and another undifferentiated partially occupied metal sites. The dehydration dynamics of stilbite was studied by Cruciani et al. (1997) using synchrotron X-ray powder diffraction.
By far the more common composition of this series is stilbite-Ca. The framework composition is moderately silica rich with Si varying between 25.3 and 28.2 per unit cell or TSi between 0.706 and 0.785. Ca and Na are the common non-framework cations and form a compositional series. K occurs rarely, while other elements like Mg, Sr, and Ba are absent or very minor in amount. As shown by Passaglia et al. (1978) there is no compositional gap between stellerite and stilbite. Typically stilbite-Ca contains four Ca per unit cell and variable amounts of Na, from near zero to greater than 2. Only a few stilbite-Na samples are known, and these have Na in excess of four and Ca between 1 and 2 cations per cell.
The H2O content of members of this series depends on the number of cations in the channels. Stilbite-Ca contains between 28 and 30 H2O molecules per cell, while stilbite-Na with 7 to 8 cations per cell has 25 to 26 H2O molecules per unit cell (Deer et al. 2004).
Diagenesis and burial metamorphism environments.
Minerals of the stilbite series are rare constituents of diagenetically altered or weakly metamorphosed volcaniclastic sedimentary rocks. Stilbite is virtually unknown in deep sea sediment samples, although traces of stilbite have been detected in merlinoite-bearing, manganese nodule samples from the Central Indian basin, Indian Ocean (Mohatapatra and Sahoo 1987).
Stilbite, presumably stilbite-Ca, occurs in widely scattered localities in the altered Tertiary volcanic sedimentary sequences in Japan (Iijima and Utada 1972). The rare occurrences of stilbite in the thermal aureole in the Tanzawa Mountains of central Japan are believed by Seki et al. (1969) to occur only in mafic host rocks in Zone I, where mordenite is characteristic in silicic protoliths.
An important, but under-reported, occurrence of stilbite is in veins cutting sedimentary rocks that have been altered to heulandite or laumontite. Two such occurrences were briefly described by Coombs (1954) in Triassic sediments of the Southland Syncline, South Island, New Zealand, and by Murata and Whiteley (1973) in Miocene sandstone formation of the Central Coast Rages, California. These fractures are obviously post-diagenesis and may represent a retrograde reaction, like heulandite (in wall-rock) + H2O = stilbite.
Murata and Whiteley 1973 observed both stilbite and heulandite replaced by laumontite in some veins. Stallard and Boles (1989) and Boles (1991) used oxygen isotope compositions of vein stilbite to estimate the temperature of formation. Assuming a vein water of about -2 per mil (a mix of marine pore water about 0 per mil and meteoric water -6 to -12 per mil) the crystallization temperature of stilbite ranges from 62° to 78°C through the 10 km of exposed section.
Diagenesis and alteration of mafic lava flows.
By far the most notable stilbite-Ca occurrences are in cavities in tholeiitic basalt. Specimens of crystals up to 10 cm have been collected from the classic localities, such as Teigarhorn and Roudafell in Berufjord, eastern Iceland; Nasik and Pune, India; Faroe Islands; Minas Basin and the Bay Fundy, Nova Scotia, Canada; the Glascow area, Scotland; and quarries in and near Paterson and Prospect Park, New Jersey, U.S.A. Descriptions, including associated minerals, of these occurrences and many more are given by Tschnerich (1992).
Few studies have explored the conditions controlling this type of occurrence of stilbite. However, several investigations of the alteration of basalt flow sequences in Iceland may be extended to other localities, in order to estimate the physicochemical conditions of stilbite crystallization. Particularly informative are those on the eastern Iceland localities (Walker 1960) and the geothermal areas near Reykjavik (Kristmannsdóttir and Tómasson 1978). Zonal distribution of zeolite species in the thick sequences of Tertiary basalt in eastern Iceland show stilbite in the lowest zone with mesolite and scolecite (Walker 1960). This zone is about 800 m thick, and the maximum burial depth of the top of the zone was about another 800 m. Similar assemblages occur in the low temperature, geothermal areas of Reykjavik at temperatures from 90° to 110°C and depths of 900 to 1000 m (Kristmannsdóttir and Tómasson 1978). These conditions show that the stilbite-bearing zone formed in a region of moderately high heat flow (65° to 125° per kilometer). Murata et al. (1987) suggest that regional zoning of zeolites in cavities of lavas occurs in regions of high heat flow, like spreading centers. Whereas localized zeolite occurrence is typical of low heat flow areas, such as in fore-arc basins.
Although stilbite-Ca is a common alteration product of basalt, its abundance is controlled by the bulk composition of the host rock. Because of its relatively high TSi content, stilbite-Ca tends to occur in tholeiitic basalt or basaltic andesite (Walker 1960, Murata et al. 1987). In Iceland, for example, the major stilbite occurrences are in the tholeiitic basalt flows, and not in the olivine basalt buried to the same depths.
Stilbite-Na is uncommon, and the known occurrences in basalt cavity localities are from sea-side localities. The type locality is at Capo Pula, Cagliari, Sardinia in altered andesite in sea cliffs (very near the type locality of barrerite). Other occurrences are in the Victorian basalt, exposed on Phillip Island, Victoria, Australia (Birch 1989) and in basalt exposed in the tidal area of Kuiu and Kupreanof Islands in southeastern Alaska (Di Renzo and Gabelica 1997). Passalia et al. (1978) and Di Renzo and Gabelica (1997) suggest that the unusually high Na contents for stilbite are related to the close proximity of the localities to sea water. It is not clear whether the Na contents result from sea water control of the altering solutions or from later ion exchange with sea water.
Hydrothermal systems.
Stilbite does not occur in rocks near many active geothermal systems, largely because the wall rocks are commonly rhyolitic and the temperatures, too high. As mentioned above, stilbite-Ca has been found in basaltic rocks of the low temperature geothermal area near Reykjavik, Iceland, where occurs in the same zone as thomsonite, heulandite, and mordenite, 90° to 110°C and depths 800 to 1000 m (Kristmannsdóttir and Tómasson 1982).
Zeolites, stilbite in particular, are rare in epithermal ore veins. A notable occurrence, however, is at the Samson Mine, Andreasberg, Harz, Germany, where stilbite-Ca (Passaglia et al. 1978, Haüy 1801) occurs with heulandite, analcime, calcite, pyrite, quartz, fluorite, and galena.
Stilbite-Ca has long been known to occur in veins and other cavities in hypabyssal and plutonic rocks. The stilbite may form from fluids passing through joints or faults during late stages of crystallization, but also from retrograde reactions during uplift events. Some such occurrences are in tonalite, Val Nambrone, Trento, Italy (Alberti 1971); with chabazite and laumontite in the granite of Baveno near Lago Maggiore, Italy (Pagliani 1948); in the Camas Land gabbro, Washington, USA, where stilbite replaces plagioclase (Chappell 1933); and with laumontite in a breccia zone in granodiorite near the scheelite ore body of the Pine Creek Mine, Bishop, California, USA (Gray et al. 1968). The surface manifestation of this environment of alteration may be represented by hot springs, like those described by Mariner and Presser (1995). Stilbite-Ca and chabazite-Ca line fractures and cement cobbles in terraces associated with hot springs in the Idaho batholith, central Idaho, U.S.A. Spring waters that precipitated these zeolites are alkaline and are at temperatures in the range 50° to 86°C (Mariner and Presser 1995). In Japan stilbite-Na occurs in cavities in a Miocene granite porphyry on the Kii Peninsula, Onigajo, Mié Pref. (Harada and Tomita 1967). Stilbite-Na forms the drusy lining of cavities and is covered by heulandite and finally by laumontite, suggesting a temperature increasing sequence.
Stilbite also occurs in granitic rocks as a late stage deuteric (hydrothermal) alteration product of feldspars or other Ca-silicates. Examples are in the pocket zones of pegmatite dikes, such as the Himalaya dike, San Diego County, California (Foord, 1977); the dike at Jensen Quarry, Riverside County, California (DeVito and Ordway, 1984); at Elveneset, Innhavet, Norway (Saebø and Sverdrup 1959); and near San Piero in Campo, Elba, Italy (Orlandi and Scortecci 1985).
Stilbite-Ca occurs as a low temperature alteration product along fractures in several kinds of metamorphic rocks, for example the alpine-clefts in the granitic gneiss of the Aar Massif, Switzerland (Parker 1922). Of the many occurrences in Switzerland described by Stalder et al. (1973) a few notable ones are Pedemonte, Bellinzona, Tessin (Gschwind and Brandenberger 1932); Gibelsbach near Fliesch (Stalder et al. 1973); and Schirstock, Fellithal, Uri (Passaglia et al. 1978). In some cases it remains questionable whether reported stilbite-Ca is really stilbite and not stellerite (e.g., Armbruster et al. 1996). Similar vein occurrences have been found in the eastern U.S., in gneiss at the Tilly Foster Mine near Brewster, New York, USA (Trainer 1938); along subway excavations in New York City (Manchester 1931); also in gneiss at Jones Falls, Maryland (Shannon 1925); and several localities in Connecticut, like in the gneiss and granite at Thachersville (Dana 1899)
Akizuki, M and Konno, H. 1985. Order-disorder structure and the internal texture of stilbite. Am. Mineral. 70, 814-821.
Akizuki, M., Kudoh, Y. and Satoh, Y. 1993. Crystal structure of the orthorhombic {001} growth sector of stilbite. Eur. J. Mineral. 5, 839-843.
Armbruster, T., Kohler, T., Meisel, T., Nägler, T., Götzinger, M.A., Stalder, H.A. 1996. The zeolite, fluorite, quartz assemblage of the fissures at Gibelsbach, Fiesch (Valais, Switzerland): crystal chemistry, REE patterns, and genetic speculations. Schweiz. Mineral. Petrogr. Mitt. 76, 131-146.
Birch, W.D. 1989. Zeolites of Victoria, Mineral. Soc. Victoria (Australia), Sp. Pub. No. 2.
Boles, J.R. 1991. Diagenesis during folding and uplift of the Southland Syncline, New Zealand. New Zealand Jour. Geol. and Geophys. 34, 253-259.
Chappell, W.M. 1933. Paulopost stilbite in the Camas Land sill, Chelan County, Washington. Am. Mineral. 18, 440-
Coombs, D.S. 1954. The nature and alteration of some Triassic sediments from Southland Syncline, New Zealand. Trans. Royal Soc., New Zealand, 82, 65-109.
Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillmanns, E., and Vezzalini, G. 1997. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral., 35, 1571-1606.
Cruciani, G., Artioli, G., Gualtieri, A., Ståhl, K., and Hanson, J.C. 1997. Dehydration dynamics of stilbite using synchrotron X-ray powder diffraction. Am. Mineral. 82, 729-739.
Dana, E.S. 1899. The system of mineralogy of James Dwight Dana. 6th Ed. John Wiley and Sons, New York, 1134 pp.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004). Rock Forming Minerals. vol. 4B.
Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites. The Geological Society, London.
DeVito, F. and Ordway, A., Jr. 1984. The Jensen Quarry, Riverside County, California. Min. Rec. 15, 273-290.
Foord, E.E. 1977. The Himalaya dike system, Mesa Grande District, San Diego County, California. Min. Rec., 8, 461-474.
Galli, E. 1971. Refinement of the crystal structure of stilbite. Acta Crystallogr. B27, 833-841.
Galli, E. and Gottardi, G. 1966. The crystal structure of stilbite. Mineral. Petrogr. Acta (Bologna) 12, 1-10.
Gray, R.F., Hoffman, V.J., Bagan, R.J. and McKinley, H.L. 1968. Bishop Tungsten District, California. Ore Deposits of the United States, 1933-1967, the Graton-Sales Volume, Ridge, J.D. (ed), 2, 1531-1554.
Gschwind, M. & Brandenberger, E. 1932. Über zwei neue Zeolithvorkommen im Tessin. Schweiz. Mineral. Petrog. Mitt., 12, 445.
Harada, K. and Tomita, K. 1967. A sodian stilbite from Onigajo, Mie Prefecture, Japan with some experimental studies concerning the conversion of stilbite to wairakite at low water vapor pressures. Am. Mineral. 52, 1438-1450.
Haüy, A.R. 1801. Traité de Minéralogie. 3, Ches Louis, Paris.
Iijima, A. and Utada, M. 1972. A critical review on the occurrence of zeolites in sedimentary rocks in Japan. Japan. J. Geol. Geogr., 42, 61-83.
Kristmannsdóttir, H. and Tómasson, J. 1978. Zeolite zones in geothermal areas in Iceland. In, Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 277-284.
Manchester, J.G. 1931. The minerals of New York City and its environs. Bull. New York Mineral. Club 3, 1-168.
Mariner, R.H. and Presser, T.S. 1995. Conditions of stilbite and chabazite formation in hot springs of the Idaho batholith, Central Idaho. In Ming, D.W. and Mumpton, F.A. (eds). Natural Zeolites ‘93, Int. Comm. Natural Zeolites, Brockport, New York, 565-577.
Mohatapatra, B.K. and Sahoo, R.K. (1987) Merlinoite in manganese nodules from the Indian Ocean. Min. Mag. 51, 749-750.
Murata, K.J., Milton, L.I., Formoso, L.L., and Roisenberg, A. 1987. Distribution of zeolites in lavas of southeastern Parana Basin, State of Rio Grande do Sol, Brazil. J. Geol., 95, 455-467.
Murata, K.J. and Whiteley, K.R. 1973. Zeolites in the Miocene Briones Sandstone and related formations of the central Coast Ranges, California. J. Res. U.S. Geol. Surv., 1, 255-266.
Orlandi, P. and Scortecci, P.B. 1985. Minerals of the Elba Pegmatites. Min. Rec., 16, 353-363.
Pagliani, G. 1948. Le zeoliti del granito di Baveno. Period. Miner. 17, 175-188.
Parker, R.L. 1922. Über einige schweizerische Zeolithparagenesen. Schweiz. Mineral. Petrog. Mitt., 2, 290-298.
Passaglia, E., Galli, E., Leoni, L. and Rossi, G. 1978. The crystal chemistry of stilbites and stellerites. Bull. Minéral. 101, 368-375.
Quartieri, S. and Vezzalini, G. 1987. Crystal chemistry of stilbites: structure refinements of one normal and four chemically anomalous samples. Zeolites, 7, 163-170.
Saebø, P.C. and Sverdrup, T.L. 1959. Note on stilbite from a pegmatite at Elvenset, Innhavet in Nordland Co., northern Norway. Norg. Geol. Unders. 205, 181-183.
Seki, Y., Oki, Y., Matsuda, T., Mikami, K. and Okumura, K. 1969. Metamorphism in the Tanzawa Mountains, Central Japan. J. Japan. Assoc. Miner Petrol. Econ. Geol., 61, 1-75.
Shannon, E.V. 1925. The so-called halloysite of Jones Falls, Maryland. Am. Mineral. 10, 159-161.
Slaughter, M. 1970. Crystal structure of stilbite. Am. Mineral. 55, 387-397.
Stalder, H.A., de Quervain, F., Niggli, E., Graeser, St. and Jenny, V. 1973. Die Mineralfunde der Schweiz, revised edition of Parker, R.L. Die Mineralfunde der Schweizer Alpen, Wepf and Co, Verlag, Basel. 433 pp.
Stallard, M.L. and Boles, J.R. 1989. Oxygen isotope measurements of albite-quartz-zeolite assemblages, Hokunui Hills, Southland, New Zealand. Clays and Clay Minerals. 37, 409-418.
Trainer, J.N. 1938. Tilly Foster up-to-date. Rocks and Minerals, 13, 291-303.
Tschernich, R.W. 1992. Zeolites of the World. Geoscience Press, Tucson. 563 pp.
Walker, G.P.L. 1960. Zeolite zones and dike distribution in relation to the structure of the basalts of eastern Iceland. J. Geol., 68, 515-528.