
Hardness: 5.
D = 2.25 - 2.29 gm/cm3.
Luster: vitreous. Streak: white
Biaxial (-). α = 1.507 - 1.513, β = 1.516 – 1.520, γ = 1.517 – 1.521. δ = 0.0.08 – 0.010.
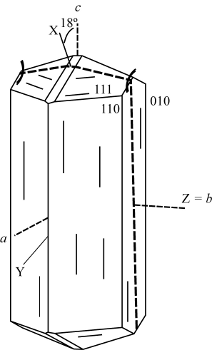
Z = b, X ˄ c. 2Vx = 36° - 56°.
Dispersion: r < v, strong
Z = 8.
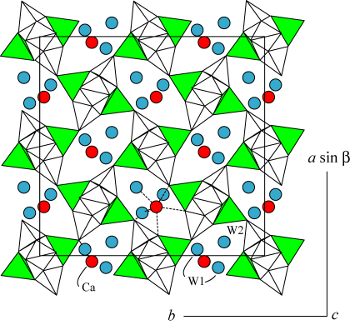
Space group: F1d1
Diagenesis and very low grade metamorphism of basalt and other kinds of lava flows.
Cavity and vein fillings in altered basaltic lavas is the setting for most occurrences of scolecite. Some of the classic occurrences are the fine sprays, 8-10 cm long in Tertiary basalt cavities at Teirgarhorn, Berufjord, Iceland (Walker 1960, Betz 1981); in the Tertiary basalt at Ben More, Isle of Mull and at Talisker Bay, Isle of Skye, Scotland (Heddle 1901); in cavities in the Deccan basalt at Nasik and Pune, India (Currier 1976); in large pockets in basalt of the Antas Railway Tunnel near Bento Goncalves, Rio Grande do Sul, Brazil (Lieber 1978). Regional occurrence of scolecite zones, like those of Iceland (Walker 1960), occur in Rio Grande do Sol, Brazil (Murata et al. 1987).
Active and fossil hydrothermal systems.
Scolecite has been found only in the Icelandic geothermal areas. Kristmannsdóttir and Tómasson (1978) define Zone 2 as the Mesolite/Scolecite Zone, probably forming in the temperature interval between 70° and 90°C. The controlling factor is the effect that the basaltic walls rocks have on the geothermal fluids from which the zeolites crystallize.
Deuteric to hydrothermal alteration.
Scolecite occurs as a low temperature alteration product along fractures in several kinds of metamorphic rocks, for example the alpine-clefts in the granitic gneiss of the Gottard and Aar Massifs, Switzerland (Parker 1922, Armbruster et al. 1996, and Weisenberger 2009). Of the many occurrences in Switzerland described by Weisenberger (2009) a few notable ones are in the walls of the Gotthard road tunnel and the Gotthard-NEAT tunnel, Gibelsbach near Fiesch (Stalder et al. 1973 and Armbruster et al. 1996); and Schirstock, Fellithal, Uri (Parker 1922).
Alberti, A., Pongiluppi, D., Vezzalini, G. 1982. The crystal chemistry of natrolite, mesolite and scolecite. Neues Jahrb. Miner. Monatsh. 1982, 231-248.
Armbruster, T., Kohler, T., Meisel, T., Nägler, T.F., Götzinger, M.A., and Stalder, H.A. 1996. The zeolite, fluorite, quartz assemblage of the fissures at Gibelsbach, Fiesch (Valais, Switzerland): crystal chemistry, REE patterns, and genetic speculations. Schweiz. Mineral. Petrogr. Mitt. 76, 131-146.
Betz, V. 1981. Zeolites from Iceland and the Faeroes. Mineral. Rec. 12, 5-26.
Currier, R.H. 1976. Production of zeolite mineral specimens from the Deccan Basalt in India. Min. Rec. 7, 248-264.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004). Rock Forming Minerals. vol. 4B.
Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites. The Geological Society, London.
Fälth, L. and Hansen, S. 1979. Structure of scolecite from Poona, India. Acta Crystallogr. 35, 1877-1880.
Foster, M.D. 1965a. Composition of zeolites of the natrolite group. U.S. Geol. Surv., Prof. Paper 504-D, 7 pp.
Foster, M.D. 1965b. Compositional relations among thomsonites, gonnardites, and natrolites. U.S. Geol. Surv., Prof. Paper 504-E, 10 pp.
Gehlen, A.F. and Fuchs, J.N. 1813. Über Werner’s Zeolith, Haüy’s Mesotype und Stilbite. (Schweigger’s) J. Chem. und Phys. 8, 353-366.
Haüy, R.-J. 1801. Traité de minéralogie 3. Chez Louis, Paris, France.
Heddle, M.F. 1901. Mineralogy of Scotland. Edinburgh, v. 2, 110-112.
Hey, M.H. 1932. Studies on the zeolites. Part III. Natrolite and metanatrolite. Min. Mag. 23, 243-289.
Joswig, W., Bartl, H. and Fuess, H. 1984. Structure refinement of scolecite by neutron diffraction. Z. Kristallogr. 166, 219-223.
Kvick, Å. and Ståhl, K. 1985. A neutron diffraction study of the bonding of zeolitic water in scolecite at 20 K. Z. Kristallogr. 171, 141-154.
Kristmannsdóttir, H. and Tómasson, J. 1978. Zeolite zones in geothermal areas in Iceland. in Natural Zeolites, Occurrence, Properties, Use, Pergamon Press, Oxford., 277-284.
Lieber, W. 1978. Das Antas, Brasilien. Lapis, 3, 24-27.
Neuhoff, P.S., Kroeker, S., Lin-Shu Du, Fridrikson, T., and Stebbins, J. (2002) Order/disorder in natrolite group zeolites: A 29Si and 27Al MAS NMR study. Am. Mineral. 87, 1307-1320.
Parker, R.L. 1922. Über einige schweizerische Zeolithparagenesen. Schweiz. Min. Petr. Mitt., 2, 290-298.
Smith, J.V., Pluth, J.J., Artioli, G., Ross, F.K. 1984. Neutron and x-ray refinements of scolecite. In Olson, D. and Bisio, A. (eds). Proceedings of the Sixth International Zeolite Conference, Reno, USA., Butterworths, 842-850.
Stalder, H.A., de Quervain, F., Niggli, E., Graeser, St. and Jenny, V. 1973. Die Mineralfunde der Schweiz, revised edition of Parker, R.L. Die Mineralfunde der Schweizer Alpen, Wepf and Co, Verlag, Basel. 433 pp.
Walker, G.P.L. 1960. Zeolite zones and dike distribution in relation to the structure of the basalts of eastern Iceland. J. Geol. 68, 515-528.
Weisenberger T. (2009) Zeolite in fissures of crystalline basement rocks. PhD thesis, University of Freiburg, 178 pp.