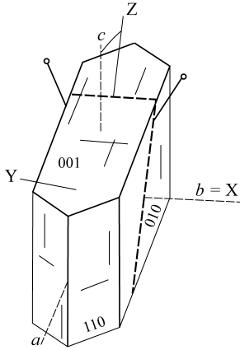
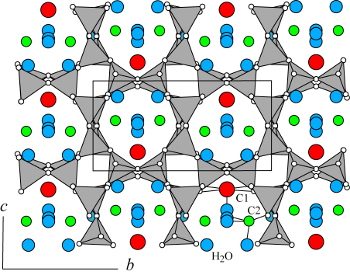
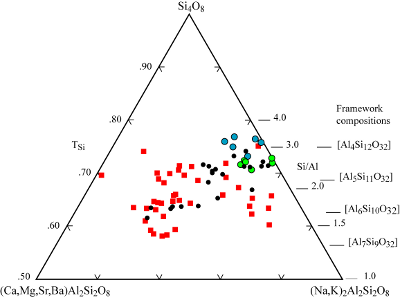
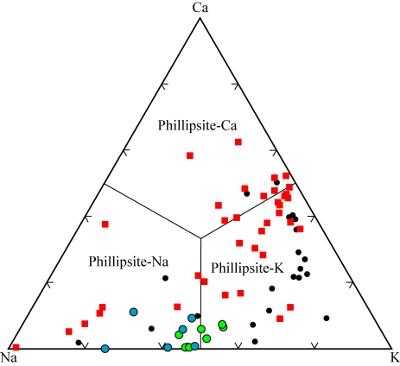
| |
Akizuki, M. 1985. The origin of sector twinning in harmotome. Am. Mineral. 70, 822-828.
Alt, J.C., Teagle, D.A.H., Brewer, T., Shanks, W.C. III and Halliday, A. 1998. Alteration and mineralization of an oceanic forearc and the ophiolite-ocean crust analogy. J. Geophy. Res. 103, 12365-12380.
Bass, M.N. 1976. Secondary minerals in oceanic basalts, with special reference to Leg 34, Deep Sea Drilling Project, in Yeats, R.S. et al., eds., Initial Reports of the Deep Sea Drilling Project, XXXIV, U.S. Gov. Printing Office, Washington, D.C., 393-432.
Bernat, M., Bieri, R.H., Koide, M., Griffin, J.J., and Goldberg, E.O. 1970. Uranium, thorium, potassium and argon in marine phillipsites. Geochim. Cosmochim. Acta, 34, 1053-1071.
Betz,V. 2005. Ein Beitrag zur Morphologie, Paragenese und Geschichte der Zeolithe vom Limberg bei Sasbach / Kaiserstuhl. Der Aufschluss, 56, 235-250.
Boettinger, J.L. and Graham, R.C. 1995. Zeolite occurrence in soil environments: an updated review. In Ming, D.W. and Mumpton, F.A. (eds). Natural Zeolites ‘93, Int. Comm. Natural Zeolites, Brockport, New York, 23-37.
Boles, J. R. 1977. Zeolites in deep-sea sediments. in Mineralogy and Geology of Natural Zeolites, Miner. Soc. Amer., Short Course Notes, 4, 137-163.
Bölke, J.K., Honnorez, J. and Honnorez-Guerstein, B.-M. 1980. Alteration of basalts from Site 396-B, DSDP: petrographic and mineralogic studies. Contr. Mineral. Petrol. 73, 341-364.
Černý, P. 1960. Milarite and wellsite from Vezne. Brnenske Zakladny Cesk. Akad. Ved, 32, 1-18.
Chauhan, O.S., Gujar, A.R. and Rao, C.M. 1994. On the occurrence of ferromanganese micronodules from the sediments of the Bengal Fan; a high terrigenous sediment input region. Earth and Plant. Sci. Letters, 128, 563-573.
Colella, C., de’Gennaro, M., and Aiello, R. 2001. Use of zeolitic tuff in the building industry. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 551-587.
Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillmanns, E., and Vezzalini, G. 1997. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 35, 1571-1606.
Czyscinksi, K. 1973. Authigenic phillipsite formation rates in the Central Indian Ocean and the Equatorial Pacific Ocean. Deep Sea Res. 20, 555-559.
de’Gennaro, M., Adabbo, M. and Langella, A. 1995. Hypothesis on the genesis of zeolites in some European volcaniclastic deposits. In Ming, D.W. and Mumpton, F.A. (eds). Natural Zeolites ‘93, Int. Comm. Natural Zeolites, Brockport, New York, 51-67.
Di Franco, S. 1942. Mineralogia Etnea. Zuccarello and Izzi, Catania, Italy. (158-161).
Galli, E. and Loschi Ghittoni, A.G. 1972. The crystal chemistry of phillipsites. Am. Mineral. 57, 1125-1145.
Gottardi, G. and Galli, E. 1985. Natural Zeolites, Springer-Verlag, Berlin, Germany. 409 pp.
Hay, R.L. 1964. Phillipsite of saline lakes and soils. Am. Mineral. 49, 1366-1387.
Hay, R.L. 1966. Zeolites and zeolitic reaction in sedimentary rocks. Geol. Soc. Amer., Spec. Pap. 85, 130 pp.
Hay, R.L. 1968. Chert and its sodium-silicate precursors in sodium-carbonate lakes of East Africa. Contr. Mineral. Petrol. 17, 255-274.
Hay, R.L. 1970. Silicate reaction in three lithofacies of a semiarid basin, Olduvai Gorge, Tanzania. Miner. Soc. Amer. Spec. Paper 3, 237-255.
Hay, R.L. 1978. Geologic occurrence of zeolites. In Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 135-143.
Hay, R.L. and Iijima, A. 1968. Petrology of palagonite tuffs of Koko craters, Oahu, Hawaii. Contr. Mineral. Petrol. 17, 141-154.
Hay, R.L. and Sheppard, R. A. 1977. Zeolites in open hydrologic systems. In Mumpton, F.A. (ed). Mineralogy and Geology of Natural Zeolites, Miner. Soc. Am., Short Course Notes, v. 4, 93-102.
Horváth, L. and Gault, R.A. 1990. The mineralogy of Mont St. Hilaire, Quebec. Min. Rec. 21, 284-359.
Kallo, D. 2001. Applications of natural zeolites in water and wastewater treatment. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 519-550.
Kastner, M. and Stonecipher, S.A. 1978. Zeolites in pelagic sediments of the Atlantic, Pacific, and Indian Oceans. In Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 199-220.
Kristmannsdóttir, H. and Tómasson, J. 1978. Zeolite zones in geothermal areas in Iceland. In Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 277-284.
Langella, A., Cappelletti, P. and De’Gennaro, M. 2001. Zeolites in closed hydrologic systems. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 235-260.
Lee, C.H. and Lee, S-R. 1998. Authigenic phillipsite in deep-sea manganese nodules from the Clarion-Clipperton area, NE Equatorial Pacific. Marine Geol., 148, 125-133.
Lenzi, G. and Passiglia, E. 1974. Fenomeni di zeolitizzazione nelle formazioni vulcaniche della regione sabatina. Boll. Soc. Geol. It. 93, 623-645.
Lévy, A. 1825. Descriptions of two new minerals. Annals. of Phil., new ser. 10, 361-363.
Linkletter, G.O. 1974. Authigenic phillipsite in Antarctic lacustrine sediments. Geol. Soc. Amer. Abstracts with Prog. 6, 206-207.
Mariner, R.H. and Surdam, R.C 1970. Alkalinity and formation of zeolites in saline alkaline lakes. Science, 170, 977-980.
Ming, D.W. and Boettinger, J.L. 2001. Zeolites in Soil Environments. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 323-346.
Ming, D.W. and Dixon J.B. 1988. Occurrence and weathering of zeolites in soil environments, In Kalló, D. and Sherry, H.S. (eds.) Occurrence, Properties and Utilization of Natural Zeolites. Akadémiai Kiadó, Budapest, 619-715.
Ming, D.W. and Mumpton, F.A. 1989. Zeolites in soils. in Minerals in Soil Environments, 2nd ed., J.B. Dixon and S.B. Weed, eds., Soil Science Society of America, Madison, Wisconsin, 873-911.
Murray, J. and Renard, A.F. 1891. Report on deep-sea deposits. Report on the Scientific Results of the Voyage of “H.M.S. Challenger” During the Years 1873-1876, Neill and Co., Edinburgh, 520 pp.
Passaglia, E., Gualtieri, A.F. and Galli, E. 2000. Variations of the physical and chemical properties in cation-exchanged phillipsites. in Colella, C. and Mumpton, F.A., eds, Natural Zeolites for the Third Millennium. De Frede Editore, Napoli, Italy. 259-267.
Passaglia, E. and Vezzalini, G. 1985. Crystal chemistry of diagenetic zeolites in volcanoclastic deposits of Italy. Contr. Mineral. Petrol. 90, 190-198.
Passaglia, E., Vezzalini, G., and Carnevali, R. 1990. Diagenetic chabazites and phillipsites in Italy: crystal chemistry and genesis. Eur. J. Mineral., 2, 827-839.
Pekov, I.V. 2000. Lovozero Massif: History, Pegmatites, Minerals. Ocean Pictures Ltd., Moscow, Russia. 484 pp.
Raybon, S.O. 1982. Lithology and clay mineral variations in the middle phase of the Paleocene Porters Creek Formation of Mississippi, M.S. thesis, University of Mississippi, University, Mississippi, 101 pp.
Rinaldi, R., Pluth, J.J., and Smith, J.V. 1974. Zeolites of the phillipsite family. Refinement of the crystal structure of phillipsite and hamotome. Acta Cryst., B30, 2426-2433.
Sadanga, R., Marumo, F., Takéuchi, Y. 1961. The crystal structure of harmotome. Acta Crystallogr. 14, 1153-1163.
Sheppard, R.A. and Gude, A.J. 3rd. 1968. Distribution and genesis of authigenic silicate minerals in tuffs of Pleistocene Lake Tecopa, Inyo County, California. U.S. Geol. Surv., Prof. Paper 597, 38 pp.
Sheppard, R.A. and Gude, A.J. 3rd. 1969. Diagenesis of tuffs in the Barstow Formation, Mud Hills San Bernardino County, California. U.S. Geol. Surv., Prof. Paper 634, 35 pp.
Sheppard, R.A. and Gude, A.J. 3rd. 1973. Zeolites and associated authigenic silicate minerals in tuffaceous rocks of the Big Sandy Formation, Mohave County, Arizona. U.S. Geol. Surv., Prof. Paper 830, 36 pp.
Sheppard, R.A., Gude, A.J. 3rd., and Griffin, J.J. 1970. Chemical composition and physical properties of phillipsite from the Pacific and Indian Oceans. Am.Mineral. 55, 2053-2062.
Sheppard, R.A. and Hay, R.L. 2001. Formation of zeolites in open hydrologic systems. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 261-276.
Stonecipher, S.A. 1978. Chemistry of deep-sea phillipsite, clinoptilolite, and host sediment. In Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 221-234.
Stuckenschmidt, E., Fuess, H. and Kvick, Å. 1990. Investigation of the structure of harmotome by X-ray (293 K, 100K) and neutron diffraction (15 K). Eur. J. Mineral., 2, 861-874.
Surdam, R.C. and Eugster, H.P. 1976. Mineral reactions in the sedimentary deposits of the Lake Magadi region, Kenya. Geol. Soc. Amer. Bull., 87, 1739-1752.
Taylor, M.W. and Surdam, R.C. (1981) Zeolite reactions in the tuffaceous sediments at Teels Marsh, Nevada. Clays and Clay Minerals 29, 341-352.
Travnikova, L.S., Grandusov, B.P., and Chizhikova, N.P. 1973. Zeolites in soils. Soviet Soil Sci., 5, 251.
Walker, G.P.L. 1960. Zeolite zones and dike distribution in relation to the structure of the basalts of eastern Iceland. J. Geol., 68, 515-528.
Weisenberger, T. and Spürgin, S. 2009. Zeolites in alkaline rocks of the Kaiserstuhl Volcanic Complex, SW Germany – New microprobe investigation and the relationship of zeolite mineralogy to the host rock. Geologica Belgica, 12, 75-91.
Wernekink (1824) Beitrag zur Naturgeschichte des Harmotoms, Annalen der Physik (Gilberts Annalen) 76, Tafel 2, 171-186.
Wernekink (1825) Über den Harmotom von Annerode bei Giessen, Zeitschrift für Mineralogie, 1825. 2. Band, 25-32
Wise, W.S. and Kleck, W.D. 1988. Sodic clay-zeolite assemblage in basalt at Boron, California. Clays and Clay Minerals 36, 131-136. |