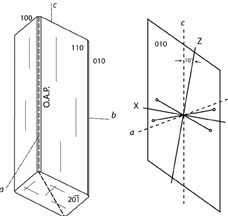
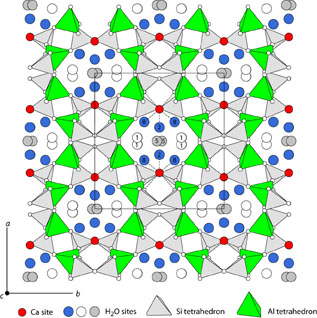
| |
Aguirre, L., Levi, B., and
Offler, R. (1978) Unconformities as mineralogical breaks in the burial
metamorphism of the Andes. Contr. Min. Petr.
66, 361-366.
Aguirre, L. and Offler, R. (1985) Burial metamorphism in the Western
Peruvian trough: its relation to Andean magmatism and tectonics. In Pitcher,
W.S., Atherton, M.P., Cobbing, E.J. and Beckinsale, R.D. (eds.) Magmatism
at a Plate Edge: the Perusian Andes, Blackie and Son Ltd, Glasgow,
59-71.
Armbrust, G.A. (1969) Hydrothermal alteration of a breccia pipe deposit,
Tribag Mine, Batchawana Bay, Ontario. Econ.
Geol. 64, 551-563.
Armbruster, T. and Kohler, T. (1992) Re- and dehydration of laumontite:
a single crystal X-ray study at 100K. Neues
Jahrb. Min., Mh. 1992, 385-397.
Artioli, G. and Ståhl, K. (1993) Fully hydrated laumontite: a structure
study by flat-plate and capillary powder diffraction techniques. Zeolites 13, 249-255.
Artioli, G., Smith, J.V., and Kvick, Å. (1989) Single crystal neutron
diffraction study of partially dehydrated laumontite at 15 K. Zeolites
9, 377-391.
Bargar, K.E., Beeson, M.H., and Keith, T.E.C. (1981) Zeolites in Yellowstone
National Park. Mineral. Rec. 12, 29-38.
Baur, W.H., Joswig, W., Fursenko, B.A., and Belitsky, I.A. (1997) Symmetry
reduction of the aluminosilicate framework of LAU topology by ordering
of exchangeable cations: the crystal structure of primary leonhardite
with a primitive Bravais lattice. Eur. J.
Mineral. 9, 1173-1182.
Blum, J.R. (1843) Leonhardite, ein neues Mineral. Poggendorff
Annalen der Physik und Chemie 59, 336-339.
Boles, J.R. (1974) Structure, stratigraphy, and petrology of mainly
Triassic rocks, Hokonui Hills, Southland, New Zealand. New
Zeal. J. Geol. Geophys. 17, 337-374.
Boles, J.R. and Coombs, D.S. (1975) Mineral reactions in zeolitic Triassic
tuff, Hokonui Hills, New Zealand. Geol. Soc.
Amer., Bull. 86, 163-173.
Boles, J.R. and Coombs, D.S. (1977) Zeolite facies alteration of sandstones
in the Southland Syncline, New Zealand. Am.
J. Sci. 277, 982-1012.
Brown, C.E. and Thayer, T.P. (1963) Low-grade mineral facies in Upper
Triassic and Lower Jurassic rocks of the Aldrich Mountains, Oregon. J.
Sed. Petrol. 33, 411-425.
Cho, M., Liou, J.G., and Maruyama, S. (1986) Transition from zeolite
to prehnite-pumpellyite facies in the Kartmutsen metabasites, Vancouver
Island, British Columbia. J. Petr.
27, 467-494.
Coombs, D.S. (1952) Cell size, optical properties and chemical properties
of laumontite and leonhardite. Am. Mineral. 37, 812-830.
Coombs, D.S. (1954) The nature and alteration of some Triassic sediments
from Southland, New Zealand. Trans. Roy. Soc.
New Zealand 82, 65-109.
Coombs, D.S., Ellis, A.J., Fyfe, W.S., and Taylor, A.M. (1959) The zeolite
facies, with comments on the interpretation of hydrothermal syntheses.
Geoch. Cosmoch. Acta 17,
53-107.
Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C.,
Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel,
E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross,
M., Sheppard, R.A., Tillmanns, E., and Vezzalini, G. (1997) Recommended
nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites
of the International Mineralogical Association, Commission on New Minerals
and Mineral Names. Can. Mineral. 35, 1571-1606.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004) Rock
Forming Minerals. vol. 4B. Framework Silicates: Silica Minerals, Feldspathoids
and the Zeolites. The Geological Society, London.
Delffs, W. (1843) Analyse
des Leonhardits. Poggendorff
Annalen der Physik und Chemie 59,
339-342.
DeVito, F. and Ordway, A., Jr. (1984) The Jensen Quarry, Riverside
County, California. Mineral. Rec. 15, 273-290.
Dickinson, W.R. (1962) Petrology and diagenesis of Jurassic andesitic
strata in central Oregon. Am. J. Sci. 260, 481-500.
Dickinson, W.R., Ojakangas, R.W., and Stewart, R.J. (1969) Burial metamorphism
of the late Mesozoic Great Valley sequence, Cache Creek, California.
Geol. Soc. Am. Bull. 80, 519-526.
Fersman, A.E. (1909) Etudes sur les zeolites de la Russia: 1. Leonhardite
et laumontite dans les environs de Simferopolis (Crimee). Trav.
du Musée géol. Pierre Le Grand, Acad. Imp. Sci. Petersbourg, Russia 2, 103-150.
Foord, E.E. (1977) The Himalaya dike stsytem, Mesa Grande District,
San Diego County, California. Mineral. Rec.
8, 461-474.
Gabuda, S.P. and Kozlova, S.G. (1995) Guest-guest interaction and phase
transitions in the natural zeolite laumontite. J.
Inclusion Phenom. Mol. Recogn. Chem. 22, 1-13.
Galloway, W.E. (1979) Diagenetic control of reservoir quality in arc-derived
sandstones: implications for petroleum exploration. In Scholle, P.A.
and Schluger, P.R. (eds.), Aspects of diagenesis,
Soc. Econ. Paleont. Mineral. Sp. Pub. 26,
251-262.
Gottardi, G. and Galli, E. (1985) Natural
Zeolites, Springer-Verlag,
Berlin, Germany. 409 pp.
Gray, R.F., Hoffman, V.J., Bagan, R.J., and McKinley, H.L. (1968) Bishop
Tungsten District, California. Ore Deposits
of the United States, 1933-1967, the Graton-Sales Volume, Ridge,
J.D. (ed.), 2, 1531-1554.
Haüy, R.-J. (1801) Traité de minéralogie 3. Chez Louis, Paris, France.
Haüy, R.-J. (1809) Tableau Comparatif des
Résultats de Cristallographie et de l’Analyse Chimique Relativement
à la Classification des Minéraux.
Courceir, Paris, France.
Hoare, J.M., Condon, W.H., and Patton, W.W., Jr. (1964) Occurrence
and origin of laumontite in Cretaceous sedimentary rocks in Western Alaska.
U.S. Geol. Surv., Prof. Pap. 501-C, C74-C78.
Horne, R.R. (1968) Authigenic prehnite, laumontite, and chlorite in
the Lower Cretaceous sediments of south-eastern Alexander Island. Brit.
Antarctic Surv. Bull. 18, 1-10.
Iijima, A. (1995) Zeolites in petroleum and natural gas reservoirs
in Japan. a review. In Ming, D.W. and Mumpton, F.A. (eds.), Natural
Zeolites ’93: Occurrence, Properties, Use. Int. Comm. Natural
Zeolites, Brockport, New York, 99-114.
Iijima, A. and Utada, M. (1972) Zeolitic zoning of the Neogene pyroclastic
rocks in Japan. Japan. J. Geol. Geog. 42, 61-83.
James, E.W. and Silver, L.T. (1988) Implications of zeolites and their
zonation in the Cajon Pass Deep Drillhole. Geophys.
Res. Letters 15,
973-976.
Jameson, R. (1805) System of Mineralogy II. Bell and Bradfute,
Edinburgh, U.K.
Jolly, W.T. (1970) Zeolite and prehnite-pumpellyite facies in south
central Puerto Rico. Contr. Min. Petr. 27, 204-224.
Kastner, M and Stonecipher, S.A. (1978) Zeolites in pelagic sediments
of the Atlantic, Pacific, and Indian Oceans. In Sand, L.B. and Mumpton,
F.A. (eds). Natural Zeolites: Occurrence,
Properties, Use, Pergamon Press,
Elmsford, New York, 199-220.
Keith, T.E.C. and Staples, L.W. (1985) Zeolites in Eocene basaltic
pillow lavas of the Siletz River volcanics, Central Coast Range, Oregon. Clays
and Clay Min. 33, 135-144.
Kiseleva, I., Navrotsky, A., Belitsky, I., and Fursenko, B.A. (1996)
Thermochemistry and phase equilibria in calcium zeolites. Am.
Mineral.
81, 658-667.
Kristmannsdóttir, H. (1982) Alteration in the IRDP drill hole compared
with other drill holes in Iceland. J. Geophys.
Res. 87, 6525-6531.
Kristmannsdóttir, H. and Tómasson, J. (1978) Zeolite zones in geothermal
areas in Iceland. In Sand, L.B. and Mumpton, F.A. (eds.). Natural
Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford,
New York, 277-284.
Levi, B. (1969) Burial metamorphism of a Cretaceous volcanic sequence
west from Santiago, Chile. Contr. Min. Petr. 24, 30-49.
Levi, A., Aguirre, L., Nyström, J.O., Padilla, H., and Vergara, M. (1989)
Low-grade regional metamorphism in the Mesozoic-Cenozoic volcanic sequences
of the Central Andes. J. Metam. Geol. 7, 487-495.
Liou, J.G. (1971) Analcime equilibria. Lithos 4, 389-402.
Liou, J.G. (1979) Zeolite facies metamorphism of basaltic rocks from
the East Taiwan Ophiolite. Am. Mineral. 64, 1-14.
Madsen, B.M. and Murata, K.J. (1970) Occurrence of laumontite in Tertiary
sandstones of the central Coast Ranges, California. Geol.
Surv. Res., U.S. Geol. Surv., Prof. Pap. 700-D,
D188-D195.
Martini, J. (1968) Etude petrographique des Gres de Traveyann entrerve
et Griffe (Haute Savoie, France). Schweiz.
miner. petrog. Mitt. 48, 539-654.
Martini, J. and Vuagnant, M. (1965) Presence du facies a zeolites dans
la formation des ‘gres’ de Traveyanne (Alpes francosuisse). Schweiz.
miner. petrog. Mitt. 45, 281-293.
McCulloh, T.H., Cashman, S.M., and Stewart, R.J. (1978) Diagenetic
baselines for interpretive reconstructions of maximum burial depths and
paleotemperatures in clastic sedimentary rocks. In D.F. Oltz,
(ed.), Low
Temperature Metamorphism of Kerogen and Clay Minerals, Soc. Econ.
Paleont. Min., Pac. Sec., Los Angeles, 18-46.
McCulloh, T.H. and Stewart, R.J. (1979) Subsurface laumontite crystallization
and porosity destruction in Neogene sedimentary basins. Geol.
Soc. Am. Abs with Prog. 11, 475.
McCulloh, T.H, Frizzel, V.A.,Jr., Stewart, R.J., and Barnes, I. (1981)
Precipitation of laumontite with quartz, thernardite, and gypsum at Sespe
Hot Spring, Western Transverse Ranges, California. Clays
and Clay Min. 29, 353-364.
Murata, K.J. and Whiteley, K.R. (1973) Zeolites in the Miocene Briones
Sandstone and related formations of the central Coast Ranges, California. J.
Res. U.S. Geol. Surv. 1, 255-266.
Obradovic, J. (1979) Authigenic minerals in middle Triassic volcanoclastic
rocks in Dinarides. Bull. Mus. Hist. Nat.
Belgrade, Ser. A 34, 13-36.
Offler, R., Aguirre, L., Levi, B., and Child, S. (1980) Burial metamorphism
in rocks of the western Andes of Peru. Lithos 13, 31-42
Orlandi, P. and Scortecci, P.B. (1985) Minerals of the Elba pegmatites. Mineral.
Rec. 16, 353-363.
Otálora, G. (1964) Zeolites and related minerals in Cretaceous rocks
of east-central Puerto
Rico. Am. J. Sci. 262, 726-734.
Pipping, F. (1966) The dehydration and chemical composition of laumontite.
Mineralogical Soc. India, Int’l Mineral. Assoc.
Volume, 159-166.
Raam, A. (1968) Petrology and diagenesis of Broughton Sandstone (Permian),
Kiama District, New South Wales. J. Sed. Petrol.
38, 319-331.
Rahn, M., Mullis, J., Erdelbrock, K., and Frey, M. (1994) Very low-grade
metamorphism of the Traveyanne greywacke, Glarus Alps, Switzerland. J.
Metam. Geol. 12, 625-641.
Rengarten, N.V. (1950) Laumontite and analcime of the Lower Jurassic
sediments of the North Caucasus. Dokl. Akad.
Nauk SSSR 70, 485-489.
Sands, C.D. and Drever, J.I. (1978) Authigenic laumontite in deep-sea
sediments. In, Sand, L.B. and Mumpton, F.A. (eds). Natural
Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 269-275.
Sawatzki, G. (1975) Étude géologique et minéralogique des flyschs à
grauwackes volcaniques du synclinal de Thônes (Haute-Savoie, France).
Arch. Sci. Genève 27.
Seki, Y., Oki, Y., Matsuda, T., Mikami, K., and Okumura, K. (1969a)
Metamorphism in the Tanzawa Mountains, Central Japan. J. Japan.
Assoc. Miner. Petrol. Econ. Geol. 61,
1-75.
Seki, Y., Onuki, H., Okumura, K., and Takashima, I. (1969b) Zeolite
distribution in the Katayama geothermal area, Onikobe, Japan. Jap.
J. Geol. Geogr. 40, 63-79.
Ståhl, K. and Artioli, G. (1993) A neutron powder diffraction study
of fully deuterated laumontite. Eur. J. Mineral. 5, 851-856.
Ståhl, K., Artioli, G., and Hanson, J.C. (1996) The dehydration process
in the zeolite laumontite: a real-time synchrotron X-ray powder diffraction
study. Phys. Chem. Minerals 23, 328-336.
Stolz, J. and Armbruster, T. (1997) X-ray single-crystal structure
refinement of Na,K-rich laumontite, originally designated ‘primary leonhardite’.
N. Jahrb. Mineral. Mh. 1997, 131-144.
Tschernich, R.W. (1992) Zeolites of the World, Geoscience Press, Phoenix,
Arizona. 563 pp.
Utada, M. (1971) Zeolitic zoning of the Neogene pyroclastic rocks in
Japan. Sci. Pap. Coll. Gen. Educ., Univ. Tokyo 21,
189-221.
Utada, M. (2001) Zeolites in burial diagenesis and low-grade metamorphic
rocks. In Bish, D.L. and Ming, D.W. (eds.) Natural
Zeolites: Occurrence, Properties, Applications, Reviews in Mineral.
and Geochem., Miner. Soc. Am. 45, 277-304.
Vergara, M., Levi, B., and Villarroel, R. (1993) Geothermal-type alteration
in a burial metamorphosed volcanic pile, central Chile. J.
Metam. Geol. 11, 449-454.
Vincent, M.W. and Ehlig, P.L. (1988) Laumontite mineralization in rocks
exposed north of San Andreas Fault at Cajon Pass, Southern California.
Geophys. Res. Letters 15, 977-980.
von Leonhard, K.C. (1821) Handbuch der Oryktognosie. Mohr and Winter,
Heidelberg, Germany.
Westercamp, D. (1981) Distribution and volcano-structural control of
zeolites and other amygdule minerals in the island of Martinique, F.W.I. J.
Volcan. Geotherm. Res. 11, 353-365.
Wise, W.S. and Moller, W.P. (1990) Occurrence of Ca-Fe silicate minerals
with zeolites in basalt cavities at Bombay, India. Eur.
J. Mineral. 2,
875-883.
Wopfner, H., Markwort, S., and Semikiwa, P.M. (1991) Early diagenetic
laumontite in the lower Triassic Manda Beds of the Ruhuhu Basin, southern
Tanzania. J. Sed. Petrol. 61, 65-72.
Wuest, T. and Armbruster, T. (2000) Type locality leonhardite: a chemical and
single-crystal X-ray study at 100K. in Colella, C. and Mumpton, F.A.. (eds.), Natural
Zeolites for the Third Millennium. De Frede Editore, Napoli, Italy, 139-150. |