
Hardness: 4.5.
D = 2.20 to 2.26 gm/cm3.
Luster: vitreous.
Streak: white.
Biaxial (-). α = 1.525 - 1.540, β = 1.531 - 1.544, γ = 1.541 - 1.550, δ= 0.010 - 0.016, 2Vx = 82 - 86°.
Z ˄ c = 42°, Y = b, O.A.P. ^ (010).
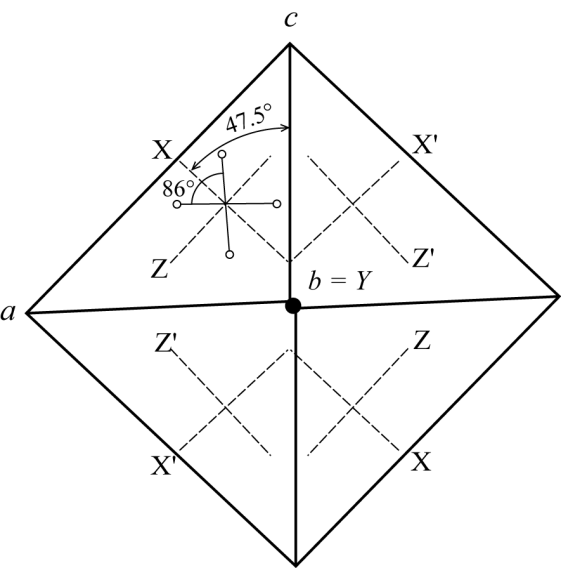
Optic orientations in twinned gismondine
a 10.023, b 10.616, c 9.843 Å, β 92.42°.
Z = 1, Space group P21/c.
(Rinaldi and Vezzalini 1985).
A Ba-dominant composition of gismondine has been found in lead-smelting slags in Yorkshire and Derbyshire (Braithwaite et al. 2001).
Some representative occurrences of gismondine in basalt cavities are the following: several localities in Austria mostly in nepheline basalt and associated with gonnardite and phillipsite; in Bohemia, Czech Republic, in basalt and leucite tephrite; various localities in Eifel District, Germany, in vesicles in nepheline basalt and in xenoliths in the basalt and is associated with phillipsite, gonnardite and many others; in vesicles of olivine basalt in eastern Iceland; the type locality is in leucitic basalt at Capo di Bova in Rome area of Italy; in olivine basalt flows of County Antrim, Northern Ireland; and in melilite nephelinite tephra of the scoria cone, Round Top, Honolulu, Hawaii.
Other types of occurrences of gismondine include miarolitic cavities in granite Brisbane, Queensland, Australia, where it is associated with prehnite, laumontite, and sulfide minerals; in metapyroxenite with other zeolites, Ontario, Canada; breccia cavities with analcime in nepheline syneite, Mont Saint Hilaire, Quebec, Canada.; and in veins and intensely sheared metaophiolitic rocks (pumpellyite-actinolite facies) and serpentinite in Liguria, Italy (Argenti et al. 1986).
Artioli, G., Rinaldi, R., Kvick, Å. and Smith, J.V. 1986. Neutron diffraction structure refinement of the zeolite gismondine at 15 K. Zeolites 6, 361-366.
Bauer, R.M. and Baur, W.H. 1998. Structural changes in the natural zeolite gismondine (GIS) induced by cation exchange with Ag, Cs, Ba, Li, Na, K and Rb. Eur. J. Mineral. 10, 133-147.
Braithwaite, R.S.W., Dyer, A., Lamb, R.P.H. and Wilson, J.I. 2001. Gismondine-Ba, a zeolite from the weathering of slags. J. Russell Soc. 7, 83-85.
Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillmanns, E., and Vezzalini, G. 1997. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral., 35, 1571-1606.
Fischer, K. and Schramm, V. 1971. Crystal structure of gismondite, a detailed refinement. In Molecular Sieve Zeolites. Am. Chem. Soc., Adv. Chem. Ser. 101. 250-258.
Gismondi, C.G. 1817. Osservazioni sopra alcuni fossili particolari de’ contorni di Roma. Giornale Enciclopedico di Napoli, Anno XI, 2, 3-15.
Kristmannsdóttir, H. and Tómasson, J. 1978. Zeolite zones in geothermal areas in Iceland. In, Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 277-284.
Leonhard, K.C. von 1817. Die Zeagonit, ein neues Mineral vom Capo do Bove bei Rom. Taschenbuch für die gesammte Mineralogie mit Hinsicht auf die neuesten Entdeckungen 11, 164-168 (from Gismondi 1817 and Coombs et al. 1997).
Milazzo, E., Artioli, G., Gualtieri, A>, and Hanson, J.C. 1998. The dehydration process in gismondine: An in situ synchrotron XRPD study. Proc. IV Convegno Nazionale Scienza e Techologica della Zeoliti, Cernobbio (Como) Italy, 160-165.
Reeuwijk, L.P. van 1971. The dehydration of gismondine. Am. Mineral. 56, 1655-59.
Rinaldi, R. and Vezzalini, G. 1985. Gismondine; the detailed X-ray structure refinement of two natural samples. In Zeolites; Synthesis, Structure, Technology and Application (eds. Drzaj, B., Hocevar, S. and Pejovnik, S.) Elsevier, Amsterdam, The Netherlands, 481-492.
Vezzalini, G. and Oberti, R. 1984. The crystal chemistry of gismondines: the non-existence of K-rich gismondines. Bull. Minéral. 107, 805-812.