Faujasite-Na |(Na,Ca0.5,Mg0.5,K)x(H2O)16|[AlxSi12-xO24] x = 3.2 – 4.3
Faujasite-Ca |(Ca0.5,Na,Mg0.5,K)x(H2O)16|[AlxSi12-xO24] x = 3.3 – 3.9
Faujasite-Mg |(Mg0.5,Ca0.5,Na,K)3.5(H2O)16|[Al3.5Si8.5 O24]
Isometric. Octahedra, {111}
Twinning on {111}, spinel law, common

Faujasite-Na octahedra with balls of phillipsite-K and offretite lining a cavity in limburgite, Sasbach, Kaisersthul, Germany. Width of view 3 mm.
Cleavage: On {111}, perfect.
Hardness: 4.5 - 5. D = 1.93 gm/cm3.
Luster: vitreous.
Streak: white.
Color: White to gray; colorless in thin section
Isotropic. n 1.466 - 1.480
Unit Cell: faujasite-Na a 24.638 (Wise, 1982) to 24.728 Å (Ibrahim and Hall, 1995)
faujasite-Ca a 24.714 to 24.783 Å (Ibrahim and Hall, 1995)
Space Group:Fd3m
Z = 16
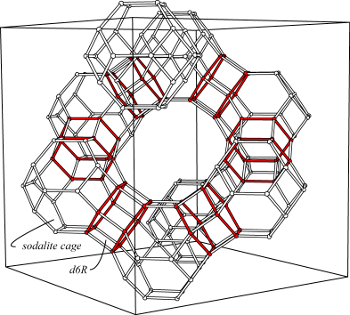 Faujasite is a rare zeolite, but is well known, because it has the same framework topology (FAU) as Linde X and Linde Y, synthetic counterparts used as sorbents and catalysts. The structure was first determined by Bergerhoff et al. (1956) and Bergerhoff et al. (1958) and was refined by Baur (1964) and by Rinaldi (pers. commun. to Gottardi and Galli, 1985). A review of structural work on Linde X and Linde Y, on ion-exchanged faujasite, and on ion-exchanged and dehydrated was provided by Smith (1988). Properties of natural and synthetic faujasite were discussed by Stamires (1973). Faujasite corresponds to the most open framework of all natural zeolites. About half of the unit-cell space is void in the dehydrated form. The structure consists of sodalite cages (see the figure and FAU) connected in a cubic manner over six-membered double rings (red d6R in the figure). Thus wide intersecting channels are formed parallel to <111> with an aperature of 7.4 Å. Approximately 50% of the cations reside in the sodalite cage bonded to three framework oxygens of the six-membered rings and additional H2O molecules. The remaining cations and H2O molecules are disordered in the large cavities.
Faujasite is a rare zeolite, but is well known, because it has the same framework topology (FAU) as Linde X and Linde Y, synthetic counterparts used as sorbents and catalysts. The structure was first determined by Bergerhoff et al. (1956) and Bergerhoff et al. (1958) and was refined by Baur (1964) and by Rinaldi (pers. commun. to Gottardi and Galli, 1985). A review of structural work on Linde X and Linde Y, on ion-exchanged faujasite, and on ion-exchanged and dehydrated was provided by Smith (1988). Properties of natural and synthetic faujasite were discussed by Stamires (1973). Faujasite corresponds to the most open framework of all natural zeolites. About half of the unit-cell space is void in the dehydrated form. The structure consists of sodalite cages (see the figure and FAU) connected in a cubic manner over six-membered double rings (red d6R in the figure). Thus wide intersecting channels are formed parallel to <111> with an aperature of 7.4 Å. Approximately 50% of the cations reside in the sodalite cage bonded to three framework oxygens of the six-membered rings and additional H2O molecules. The remaining cations and H2O molecules are disordered in the large cavities.The range of framework compositions of natural faujasite is quite narrow in comparison to those that have been synthesized. TSi varies from 0.725 to 0.680 for all but one natural faujasite, which is 0.644. Faujasite has been synthesized throughout the compositional range from near 0.57 (Linde X) to 0.708 (Linde Y) and as high as 0.75 (Robson 1989).
The non-framework cation content of the faujasite series extends from Ca-dominant to Na-dominant compositions. Mg occurs in minor amounts in all samples, and Mg-dominant faujasite has been found only in one old museum sample from Sasbach, Germany. With few exceptions K, Sr, and Ba are generally either absent or minor in the known samples.Faujasite occurs in slightly altered basaltic rocks and palagonite tephra, and hydrothermally altered granitic or metamorphic rocks.
Diagenesis or other alteration of basaltic lava flows
At the type locality (the Sasbach quarry at the Kaiserstuhl, Baden, Germany) faujasite-Na occurs in cavities of limbergite, a glassy basaltic rock with phenocrysts of olivine and augite. Faujasite-Na octahedra, covered with an amorphous coating, grow on cavity walls and are associated with phillipsite-K tufts, and offretite (Rinaldi et al., 1975). Faujasite-Ca with chabazite, phillipsite, and offretite also occur in limburgitic rocks at Attenburg, Annerod, and Haingrabenth all near Grossen-Buseck, Vogelsberg, and at Stempel, Hessen, Germany (Hentschel, 1980; Wise, 1982).
Iijima and Harada (1969) report the occurrence of faujasite-Na in a zeolite assemblage resulting from the alteration of palagonitic alkali basalt and melilite nephelinite tephra cones and tuff of the Pleistocene Honolulu Series, Oahu, Hawaii. Associated with faujasite-Na are phillipsite-Ca and -K, gismondine, chabazite-Na, gonnardite, natrolite, and analcime. All occur very near the present day ground surface and must have resulted from the alteration of the tephra by meteoric water. A similar occurrence of faujasite-Na is in the palagonitic portion of an early Pliocene tephra cone of alkali olivine basalt near Halloran Summit, San Bernardino County southeastern California, USA (Wise, 1982). Here faujasite and phillipsite are restricted to the part of the cone saturated by ephemeral ponded stream water. Ibrahim and Hall (1995, 1996) describe the occurrence of faujasite-Na and faujasite-Ca with phillipsite and chabazite in Quaternary palagonitic tuff in three areas, Tell Rimah, Jabal Aritayn, and Jabal Hannoun, of northeast Jordan. The composition of tuff varies from alkali basalt to basanite, and appears to have been altered by percolating meteoric water.
Faujasite-Na occurs with phillipsite-Na and chabazite-Na in glassy vesicular basalt at Aci Reale and at Aci Castello, on the flanks of Mt. Etna, Catania, Sicily (Wise, 1982; Gottardi and Galli, 1985).
A single occurrence of faujasite-Na was found in the core from drill hole 501 Deep Sea Drilling Project Legs 68, 69, and 70 into basalt of the Costa Rica Ridge (Kurnosov et al., 1983). It is from a depth of 293 m below the sea floor and occurs with smectite and phillipsite. Butler and Burbank (1929) report faujasite in uppermost temperature zone zeolite assemblage occurring in Precambrian vesicular basalt exposed by the Copper Falls Mine, Keweenaw County, Michigan.
Hydrothermally altered rocks
Faujasite occurs in veins of apparent hydrothermal alteration cutting Precambrian in metapyroxenite near Davis Hill and Khartoum, Ontario, Canada (Velthuizen, 1991). It is associated with chabazite, phillipsite, gismondine, mesolite, and thomsonite among others. Similarly it occurs in hydrothermal veins cutting Precambrian metapyroxenite near Laurel; Hicks Bridge and Notre Dam de la Salette, Quebec (Velthuizen, 1991). Hoffman (1901) reported faujasite with quartz and fluorite in the Daisy Mica Mine, Ottawa County Quebec. It is also associated with other zeolites in veins cutting in granitic rocks of the Aar and St. Gottard Massifs, Switzerland (Parker, 1922).
.
Baur, W.H. (1964) On the cation and water positions in faujasite. Am. Miner. 49, 697-704.
Bergerhoff, G., Koyama, H., Nowacki, W. (1956) Zur Kristallstrukturen des Faujasits. Experimenta (Basel) 12, 418-419.
Bergerhoff, G., Baur, W.H., Nowacki, W. (1958) Über die Kristallstrukturen des Faujasits. Neues Jahrb. Miner. Monatsh. 1958, 193-200.
Butler, B.S. and Burbank, W. S. (1929) The copper deposits of Michigan. U.S. Geol. Surv., Prof. Pap. 144, 238 pp.
Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillmanns, E., and Vezzalini, G. (1997) Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Min. 35, 1571-1606.
Damour, M. (1842) Description de la faujasite, nouvelle espèce minérale. Annales des Mines, Sér. 4, 1, 395-399.
Gottardi, G. and Galli, E. (1985) Natural Zeolites, Springer-Verlag, Berlin, Germany. 409 pp.
Hentschel, G. (1980) Weitere Offretit-Vorkommen im Vogelsberg (Hessen). Geol. Jahrb. Hessen 108, 171-176.
Hoffman, G.C. (1901) On some new mineral occurrences in Canada. Am. J. Sci. 152, 447-448.
Ibrahim, K. and Hall, A. (1995) New occurrences of diagenetic faujasite in the Quaternary tuffs of northeast Jordan. Eur. J. Mineral. 7, 1129-1135.
Ibrahim,K. and Hall, A. (1996) The authigenic zeolites of the Aritayn Volcaniclastic formation, north-east Jordan. Min. Deposita 31, 514-522.
Iijima, A. and Harada, K. (1969) Authigenic zeolites in zeolitic palagonitic tuffs on Oahu, Hawaii. Am. Miner. 54, 182-197.
Kurnosov, V.B., Kholodkevich, I.V., Chubarov, V.M., and Shevchenko, A.Ya. (1983) Secondary minerals in basalt from the Costa Rica Rift, Holes 501 and 504B, Deep Sea Drilling Project Legs 68, 69, and 70. Initial Rep. Deep Sea Drilling Project 69, 573-583.
Parker, R.L. (1922) Über einige schweizerische Zeolithparagenesen. Schweiz. Miner. Petr. Mitt. 2, 290-298.
Rinaldi, R., Smith, J.V., and Jung, G. (1975) Chemistry and paragenesis of faujasite, phillipsite and offretite from Sasbach, Kaisersatuhl, Germany. Neues Jahrb. Miner. Monatsh. 1975, 433-443.
Robson, H.E. (1989) High-silica faujasite by direct synthesis. In Occelli, M.L. and Robson, H.E. (eds.), Zeolite Synthesis, Am. Chem. Soc. Symp. Ser. 398, Washington., 1989, p. 436-447.
Smith, J.V. (1988) Topochemistry of zeolites and related materials. 1. Topology and geometry. Chem. Rev. 88, 149-182.
Stamires, D.N. (1973) Properties of the zeolite, faujasite, substitutional series: A review of new data. Clays & Clay Minerals. 21, 379-389.
Velthuizen, G.V. (1991) The occurrence of zeolites in the central metasedimentary belt of the Grenville Province. Rocks and Minerals 66, 44-45.
Wise, W.S. (1982) New occurrence of faujasite in southeastern California. Am. Miner. 67, 794-798.