
Cleavage: {010} perfect.
Hardness: 4. D = 2.22 - 2.28 gm/cm3
Luster: vitreous.
Streak: white
Color: Colorless, white, pinkish; colorless in thin section. Biaxial ( - )
α = 1.485 - 1.505, β = 1.497 – 1.515, γ = 1.497 – 1.519. δ= 0.012 – 0.014. 2VX = 45°. b = Y, c ˄ Z ≈ 10°, O.A.P. = (010)
Sheaves of epistilbite in hydrothermally altered basalt from Puu O Ehu quarry, Lanakai Hills, Kailua, Oahu. Hawaii. Width of view 5 mm.
124.66°
Z = 1. Space group: C2
more commonly: a 9.083, b 17.738, c 10.209 Å,
α 89.95°, β 124.58°, γ 90.00°
Z = 1. Space Group: C1
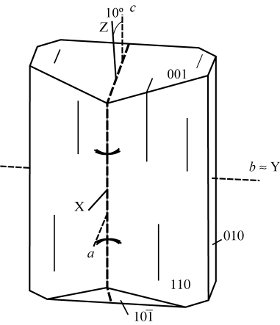
Epistilbite was described by Rose (1826) as a new member of the zeolite group, referring to material collected in Iceland and Faroe Islands at type materials. The name is from Greek epi, meaning near, and stilbite, implying a similarity to stilbite.
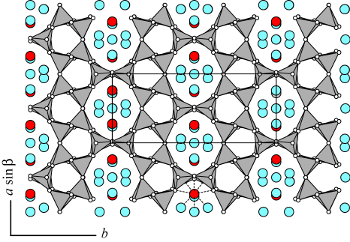
The structural framework of epistilbite possesses the same up and down orientation of tetrahedra within the sheets of six-membered rings as in dachiardite. The sheets parallel to (010), which define the perfect (010) cleavage, are also connected by four-membered rings (EPI). However, in the c direction the four-membered rings alternate at different x levels (shifted by ½) and block the ten-membered rings. Open channels are confined by eight-member rings (aperture 3.7 x 5.2 Å) extending parallel to [001] (see the accompanying figure). In triclinic epistilbite, four Ca positions (red) are located in a cage confined by the ten-membered rings of tetrahedra. Two of these sites are related to the other two sites by a pseudo two-fold axis: thus, only sites can be occupied simultaneously because of the short Ca-Ca distances. Ca has a square antiprismatic coordination with five H2O molecules and three framework oxygens (see center of bottom edge of the figure). A strong correlation exists between the Al distribution in the neighboring tetrahedra and the occupancy of the four possible Ca sites.
The compositional range of epistilbite varies from 18.5 to 17.5 Si per unit cell, and Ca comprises 65% to 90% of the non-framework cations. Ba and K are minor components in all analyses. The average H2O content from most analyses is 15.5 weight percent, which corresponds to 16 H2O molecules per unit cell.
Significant occurrences of epistilbite are in cavities of silica saturated lavas, for example, tholeiitic basalt or andesite. Some of these occurrences are clearly related to hydrothermal activity; while others are not. Epistilbite is also a product of late stage hydrothermal alteration of granitic rocks, particularly pegmatite dikes. The most common associated zeolites are heulandite, stilbite, and mordenite.
Diagenesis or other alteration of basaltic lava flows
Epistilbite, stilbite-Ca, heulandite-Ca, and scolecite occur in the zone from sea level to an altitude of 300 m in the tholeiitic basalt flows of eastern Iceland (Walker, 1960). The well known locality of Teigarhorn on the south shore of Berufjördur is in this zone (Betz, 1981), which is the lowest of the exposed zones. The zonation of zeolites in eastern Iceland is controlled by depth of burial and regional heat flow, and varies from diagenetic alteration into very low grade metamorphism of basalt flows (Mehegan et al., 1982; Kristmannsdóttir, 1982).
Epistilbite occurs in several localities in the Deccan basalt of western India, where it is associated with chabazite, laumontite, prehnite, and babingtonite among others at the Khandivali quarry north of Mumbai. Wise and Moller (1990) have shown that these assemblages crystallized at about 190°C and at an estimated depth of 2000 m. Epistilbite has also been found in basalt at Aklahare and at Pandulane near Nasik, in basalt quarries near Pune, and in the Aurangbad District (Tschernich, 1992). The conditions, under which it formed in these latter localities, are not well known.
Several notable localities in early Tertiary volcanic rocks of southwestern Washington and northwestern Oregon, USA, have produced epistilbite (Tschernich 1992). At Goble, Columbia County, Oregon, it occurs on mordenite and with heulandite and garronite in vesicles of Eocene basalt. Along the north side of Riffe Lake near Morton, Lewis County, Washington, rare 1 to 2.5 cm long epistilbite prisms occur on mordenite and quartz in vesicular basaltic rocks. Several generations of epistilbite occur in vesicular basalt near Yacolt, Clark County, Washington. Associated minerals include mordenite, heulandite, stilbite, and thomsonite. Although conditions of zeolite formation have not been determined for any of these localities, all are in a region in which early Tertiary volcanic rocks that have been buried sufficiently to have been affected by burial diagenesis or zeolite facies metamorphism.
As one would expect from the distribution of epistilbite in eastern Iceland, it also has been found in drill cores from geothermal areas, especially those with basalt host rock. Kristmannsdóttir and Tómasson (1978) report that the temperature of epistilbite formation is between 80° and 120° C, about the same as stilbite, but at lower temperatures than the laumontite zone. It is commonly associated with stilbite, heulandite, thomsonite, and mordenite.
An example of a fossil hydrothermal system in altered basalt is the area around Kailua, northeast Oahu, Hawaii. Because this area is within the former conduit system of the 2.6 Ma Koolau volcano, it is likely that the pervasive rock alteration and zeolite formation is a result of hydrothermal activity when the volcano was active. Epistilbite with chabazite, heulandite, laumontite, and mordenite occur in cavities and veins in basalt exposed in the Keolu Hills and Lanikai Hills near Kailua (Dunham, 1933).
Epistilbite and several other zeolites occur is alteration veins from late stage magmatic or metamorphic fluids. One such occurrence is the late deuteric alteration of pegmatite dikes, and well described examples are the dikes cutting the Miocene granodiorite intrusion, Monte Capanne at Forte del Prete near San Piero, Campo, Elba, Italy. Although these pegmatite dikes are well known for their tourmaline, late alteration has produced such zeolites as epistilbite, dachiardite (type locality), mordenite, stilbite, and heulandite (Galli and Rinaldi, 1974; Orlandi and Scortecci, 1985).
Some joint surfaces in gneiss have coatings of late forming minerals that include epistilbite with heulandite at Giebelsbach, Fiesch, in the Rhone Valley, and at Binntal, Valais, Switzerland (Stalder et al., 1973).
Veins cutting calc-silicate rock at Noate Mezola, Sondrio, Italy, contain epistilbite with scolecite, laumontite, and heulandite (Cerio, 1980). It is also occurs with scolecite at the contact between cal-silicate rock and granitic intrusion at the Crestmore quarry, Riverside County, California, USA (Woodford et al., 1941).
Alberti, A., Galli, E., and Vezzalini, G. (1985) Epistilbite: an acentric zeolite with domain structure. Z. Kristallogr. 173, 257-265.
Akizuki, M. and Nishido, H. (1988) Epistilbite: symmetry and twinning. Am. Miner. 73, 1434-1439.
Betz, V. (1981) Zeolites from Iceland and the Faeroes. Mineral. Rec. 12, 5-26.
Cerio, V. (1980) Le zeoliti nelle metamorfiti carbonatiche di San Giorgio (Novate Mezzola, Sondrio). Natura (Milan) 71, 118-120.
Dunham, K.C. (1933) Crystal cavities in lavas from the Hawaiian Islands. Am. Mineral. 18, 369-385.
Galli, E. and Rinaldi, R. (1974) The crystal chemistry of epistilbites. Am. Mineral. 95, 1055-1061.
Kerr, J.S. (1964) Structure of epistilbite. Nature, 202, 589.
Kristmannsdóttir, H. (1982) Alteration in the IRDP drill hole compared with other drill holes in Iceland. Jour. Geophys. Res. 87, 6525-6531.
Kristmannsdóttir, H. and Tómasson, J. (1978) Zeolite zones in geothermal areas in Iceland. In Sand, L.B. and Mumpton, F.A. (eds.). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, p. 277-284.
Mehegan, J.M., Robinson, P.T., and Delaney, J.R. (1982) Secondary mineralization and hydrothermal alteration in the Reydarfjordur drill core, eastern Iceland. Jour. Geophys. Res. 87, 6511-6524.
Merlino, S. (1965) Struttura dell’epistilbite. Atti Soc. Toscana Sci. Nat. A 72, 480-483.
Orlandi, P. and Scortecci, P.B. (1985) Minerals of the Elba pegmatites. Mineral. Rec. 16, 353-363.
Perrotta, A.J. (1967) The crystal structure of epistilbite. Mineral. Mag. 36, 480-490.
Rose, G. (1826) Ueber den Epistilbit, eine neue zur Familie der Zeolithe gehörige Mineralgattung, (Poggendorff’s) Annalen der Physik und Chemie 6, 183-190.
Slaughter, M. and Kane, W.T. (1969) The crystal structure of a disordered epistilbite. Z. Kristallogr. 130, 68-87.
Stalder, H.A., de Quervain, F., Niggli, E., Graeser, St., and Jenny, V. (1973) Die Mineralfunde der Schweiz, revised edition of Parker, R.L. Die Mineralfunde der Schweizer Alpen, Wepf and Co, Verlag, Basel. 433 pp.
Tschernich, R.W. (1992) Zeolites of the World, Geoscience Press, Phoenix, Arizona. 563 pp.
Vezzalini, G. (1984) A refinement of Elba dachiardite; opposite acentric domains simulating a centric structure. Z. Kristallogr. 166, 63-71.
Walker, G.P.L. (1960) Zeolite zones and dike distribution in relation to the structure of the basalts of eastern Iceland. J. Geol. 68, 515-528.
Wise, W.S. and Moller, W.P. (1990) Occurrence of Ca-Fe silicate minerals with zeolites in basalt cavities at Bombay, India. Eur. J. Min. 2, 875-883.
Woodford, A.O., Crippen, R.A., and Garner, K.B. (1941) Section across Commerical Quarry, Crestmore, California. Am. Mineral. 26, 887-895.
Yang, P. and Armbruster, T. (1996) (010) disorder, partial Si,Al ordering, and Ca distribution in triclinic (C1) epistilbite. Eur. J. Mineral. 8, 263-271.