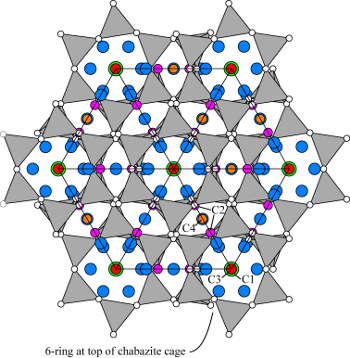
| |
Originally known only from cavities in basaltic rocks, chabazite has been widely found in altered pyroclastic rocks. It replaces rhyolitic vitric tuff in lacustrine beds from saline, alkaline lakes, as well as trachytic tuff in Italy and other places. Some rare, but informative, occurrences of chabazite, occur in altered basaltic rocks in deep marine sediment of trench margins, in shallow-level alteration of pillow basalt in ophiolite sequences, and geothermal systems hosted in basalt. The following summary is based largely on Deer et al. (2004).
Diagenesis and burial metamorphism of sediment and sedimentary rocks.
Chabazite was first discovered in sedimentary rocks by Hay (1964) in tuff and tuffaceous clay in the Olduvai Gorge, Tanzania. Since that time chabazite has been found as an authigenic alteration product in several kinds of sedimentary rocks: 1) as replacement of rhyolitic tuff interbedded with lacustrine sediment in the western U.S. and Kenya, as well as Tanzania; 2) as replacement of rhyolitic tuff beds within the marine, flysch sequence comprising the Waitemata Group, North Island, New Zealand; 3) extensive replacement of phonolitic to trachytic ignimbrite and tuff in Italy, Germany, and Canary Islands; and 4) in diamictite of the dry valleys, Antarctica.In terrestrial accumulations of volcaniclastic sediment and rock, the chabazite minerals are alteration products in some pyroclastic beds in hydrologically closed systems and in tephra and ignimbrite in hydrologically open systems. Chabazite forms early, commonly with phillipsite, replacing glass or growing as glass dissolves in interstitial water.
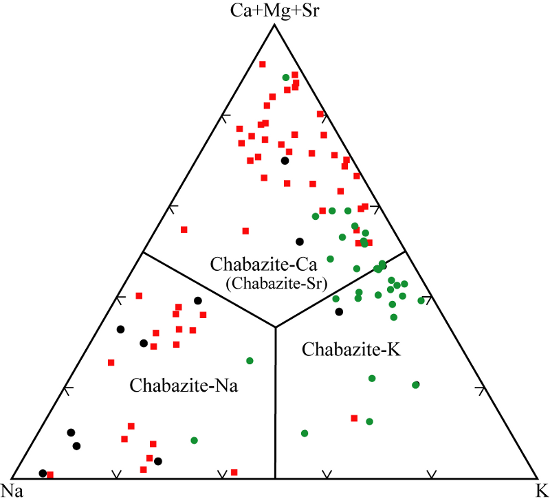
Hydrologically closed systems - tuff in lacustrine sediment. Rhyolitic, vitric tuff within lacustrine sequences from many interior valleys of the western U.S., eastern Europe, Turkey, and other localities have been replaced by authigenic zeolites, clay, and feldspar. Clinoptilolite and analcime are the most common zeolites forming in this environment, but the chabazite minerals do occur in many localities, some in economically important quantities. This type of occurrence of authigenic chabazite was first described by Gude and Sheppard (1966) and Sheppard and Gude (1969) from exposures in the Barstow Formation in southeastern California, USA. The Barstow Formation consists of 1000 to 1300 m of folded and faulted Miocene fluviatile and lacustrine rocks, exposed in the Mud Hills, northwestern San Bernardino County, California. Included in this sequence are several rhyolitic tuff beds, five of which crop out across much of the exposure area. Minerals replacing the tuff include chabazite, associated with smectite, clinoptilolite, erionite, and analcime, and potassium feldspar. Mineral facies that vary laterally along the length of exposure are a) non-analcimic zeolitic tuff, consisting of clinoptilolite, phillipsite, chabazite, erionite, and mordenite in varying proportions, b) analcimic tuff, and c) potassium feldspar-rich tuff. Chabazite varies from sparse to forming the major proportion of the beds. The species is chabazite-Na, which occurs as aggregates of anhedral crystals that are 0.002 to 0.05 mm across.Similar beds with authigenic chabazite have been described near Bowie, Cochise County (Sand and Regis 1966), and in the Pliocene Big Sandy Formation, Mohave County, Arizona (Sheppard and Gude 1973). In the latter deposit chabazite-K forms nearly monomineralic beds with lateral extents of hundreds of meters. Like in the Barstow Formation the chabazite is associated with smectite, clinoptilolite, and erionite in a nonanalcimic facies. Chabazite has not been recognized in association with opal or mordenite. It occurs as aggregates of equidimensional crystals 2 to 40 µm, and precursor shard shapes are commonly evident. Some other occurrences in the western U.S. are the Miocene lacustrine beds near Harney Lake southeastern Oregon. Here chabazite occurs mainly in southern part of basin and may comprise up to 70% of a tuffaceous bed (Sheppard 1994). The lacustrine facies of the Gila Conglomerate, possibly of Pliocene age, near Buckhorn, Grant County, New Mexico, contains a fall-out tuff mostly replaced by zeolites. Chabazite-Ca is the main zeolite in the lake-margin zone, with clinoptilolite and analcime the key minerals in the next two inward zones (Gude and Sheppard 1988). In Nevada Pliocene lake beds of the Eastgate Deposit, Churchill County and the Reese River Deposit, Lander County contain tuff beds replaced by mostly by clinoptilolite and erionite, and lesser amounts of chabazite (Papke 1972). Similar occurrences of chabazite have been described by Hay (1964 and 1970) at Olduvai Gorge, Tanzania. The three lithofacies comprising the Pleistocene deposits in Olduvai Gorge are lake deposits, lake-margin deposits, and alluvial deposits. Chabazite-Na, associated with analcime and phillipsite-Na, occurs most abundantly in thin veins cutting alluvial claystone and replacing interbedded trachytic tuff. The alluvial sediment reacted with pore fluids chemically similar to those of saline, alkaline lakes. In the hot, arid climate the soil fluids become saline and alkaline through evaporative pumping, and produce similar diagenetic products (Hay 1970). Trachytic glass readily altered to zeolites in the Oloronge Beds (Pleistocene) and High Magadi Beds (Holocene) alkaline lacustrine deposits in the Lake Magadi region, Kenya (Surdam and Eugster 1976). Erionite is the primary alteration product, with chabazite, clinoptilolite, mordenite, and phillipsite as minor associated phases. Over time these early formed phases are replaced by analcime.
Soil and surficial deposits. Chabazite occurs in some soils, developed from zeolite-bearing parent materials (Ming and Boettinger 2001), especially in arid environments. Reported occurrences are in the vicinity of Olduvia Gorge, Tanzania (Hay 1970, 1978) and in the Wright Valley in Antarctica (Gibson et al. 1983).
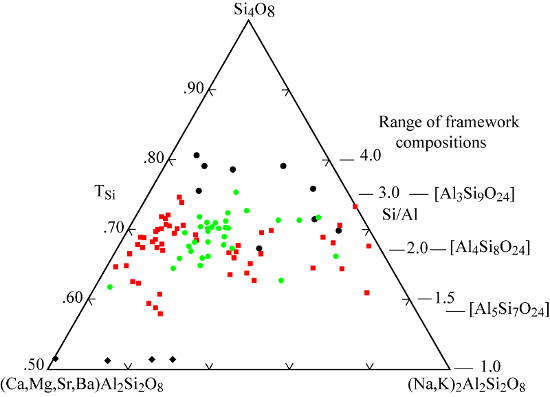
Hydrologically open systems. Terrestrial accumulations of pyroclastic debris, especially tephra and ignimbrite units, may alter to produce zeolites. Because the zeolites occur largely from reactions with through flowing vadose and groundwater, this type of process is called hydrologically open alteration (Hay and Sheppard 1977 and Sheppard and Hay 2001).In central Italy many pyroclastic deposits have been altered to zeolite, mostly chabazite-Ca, chabazite-K, and phillipsite. Some of the zeolitic units are tens of meters thick and contain up to 80% zeolite, and thereby have economic importance. The mineralogy of these deposits has been the subject of many papers following the initial discovery of the zeolites. More recent ones that include chemical analyses are by Sersale (1978), Gottardi and Obradovic (1978), Passaglia and Vezzalini (1985), Passaglia et al. (1990), de’Gennaro et al. (1995), and de’Gennaro et al. (2000). The pyroclastic units were emplaced as pyroclastic flows, ash falls, and mudflows. Compositions of the parent magmas are potassic and range from basanite to phonolite and trachyte. Even with this variety of rock types and origin, the kinds of authigenic minerals are restricted. Chabazite and phillipsite are by far the most abundant zeolites, and the compositional range of both zeolites is limited. For chabazite Ca and K are dominant non-framework cations, and TSi is in the range 0.65 to 0.75. The variation in extent and distribution of zeolitization has resulted in several different interpretations of the paragenesis.Passaglia et al. (1990) compare compositions of chabazite and phillipsite with parent glass, and consider two kinds of reactions: a) hydrologically open systems in which near neutral meteoric water yields chabazite and phillipsite with Si/Al and non-framework cations similar to parent glass, and b) mildly alkaline, saline waters in marine environments that yield zeolites with higher Na contents regardless of the parent glass composition.The tufo litoide a scorie nere is a distinctive ignimbrite exposed in the area around the lakes of Bolsena, Vico, and Bracciano in the Latium region north of Rome. It varies from a few meters to 80 m in thickness, and is nearly everywhere altered to chabazite (Lenzi and Passaglia 1974). The lack of zeolitic alteration of fall-out tuff beds of the same volcanic sequence suggests that something about the ignimbrite that makes it susceptible to the zeolitization process. The mechanism proposed has been called “geoautoclave”, in which the ignimbrite is thought to trap surface water during emplacement, starting zeolitization during cooling. A review of the mechanism and inherent difficulties is provided by Langella et al. (2001).Tufo lionato exposed southeast of Rome shows uneven zeolite distribution and variable compositions suggests alteration within a hydrologically open system. Other examples of tuff with abundant chabazite developed in open systems are the laharic units from Roccamonfina Volcano (west of Naples), the ash flow tuff erupted 30 ka in the Campanian region, and the Ercolano tuff erupted from Vesvius 79 A.D. Passaglia et al. (1990) suggest that these and similar units were altered at near surface conditions. De’Gennaro and Franco (1988) consider the temperatures of formation to have been near 100°C, based on the temperature of emplacement of the tuff units and on the observation that reactions can be correlated to tuff produced by pheatomagmatic eruptions (see below). Examples of the influence of sea water on authigenic reactions are the hyaloclastites of Vivara Island (Campania) and near Palagonia (southern Sicily). Chabazite-Na developed at Vivara, and chabazite-Ca, at Palagonia (Passaglia et al. 1990).Several aspects of the zeolite distribution in the Neapolitan Yellow Tuff, near Naples, Italy, cause de’Gennero et al. (2000) to propose an origin different from hydrologically open system alteration. The tuff originated from the nearby caldera of Campi Flegrei 12,000 years ago. Extensive zeolitic alteration has occurred in pods near the middle of the tuff, and diminishes toward the top, bottom, and distance from the source. The alkali-trachytic glass is altered to phillipsite-K, chabazite-K, and analcime. De’Gennero et al. (2000) propose that the tuff was deposited from phreatomagmatic eruptions, and zeolitic alteration occurred in those parts of the tuff near the source caldera, where residual heat and moisture could be trapped and held. This process is similar to the “geoautoclave” mechanism, in which alteration to zeolite occurs during the initial cooling of the pyroclastic deposit.Authigenic chabazite occurs as rhombs attached to the sides of pore spaces of diamictite of the Sirius Group, Table Mountain, Dry Valleys, Antarctica. Dickinson and Grapes (1997) suggest that the chabazite grew in a brine film when ice melts.
Deep Marine Sediment. Authigenic zeolites occur in most drill core from deep sea sediment in all of the oceans. Phillipsite and clinoptilolite are by far the most common, and chabazite occurs only rarely. One such occurrence is in Early middle Miocene volcanic sandstone and conglomerate from Hole 841 (Leg 135 of the Ocean Drilling Program) in the Tonga Trench Margin, Southwest Pacific Ocean (Vitali et al. 1995). At depths of about 500 m below the seafloor chabazite of unknown composition occurs with erionite and heulandite. Much of the core contains phillipsite in the uppermost 250 m, and analcime between 250 and 470 m growing in response to the thermal effects of several basaltic andesite sills.
Diagenesis of marine sediment from arc-source terrains. Chabazite is not a component of diagenetic products in most volcaniclastic sediment near island arcs. However, thin, vitric tuff beds in Miocene Waitemata Group, North Island, New Zealand, are almost completely replaced by chabazite (Sameshima 1978). Exposures are at Takapuna Beach and Karake Bay both in the Auckland City area. Chabazite is also in the tuff beds from the Kaipara region and from Parnell Grit, Auckland. These units are included in a flysch sequence, and the thickness of the whole Waitemata Group is about 1000 m. With no evidence of overlying sediment, heat to drive authigenic replacement is hypothesized to be from wide spread hot spring activity (Sameshima 1978).
Very low-grade metamorphism and the zeolite facies. Common minerals in the zeolite facies developed by burial metamorphism are laumontite and analcime. Chabazite occurs rarely, and where it does occur it is mostly in weakly metamorphosed basaltic rocks, such as seafloor pillow lavas or dikes, rather than in volcaniclastic sediment. The metamorphosed Horokanai Ophiolite was tectonically emplaced in the Kamuikotan Zone, Hokkaido, Japan. Prograde metamorphism has produced four mineral facies zones, ranging from zeolite to granulite facies (Ishizuka 1985). The zeolite zone, affecting mostly pillow lavas, is divided into three subzones with the key minerals, chabazite, laumontite, and wairakite, respectively. Assemblages of the chabazite subzone are chlorite+chabazite+ analcime+thomsonite and chlorite+chabazite+analcime+stilbite. The next higher subzone typically contains laumontite-bearing assemblages. The chabazite species was not determined but is likely to be chabazite-Ca. Ishizuka (1985) interprets the assemblages originating through very low-pressure, ocean-floor metamorphism. From a similar setting Liou (1979) reports chabazite in the assemblage of zeolites filling veins and amygdaloidal cavities in the pillow lavas of the East Taiwan ophiolite. Others are heulandite, laumontite and thomsonite.
Diagenesis and low-grade metamorphism of mafic lava flows.
Chabazite-Ca and chabazite-Na are common in cavities of basaltic rocks, most commonly associated with phillipsite, gmelinite, levyne, analcime, and heulandite. A few of the many well known localities are in eastern Iceland (Walker 1960), the Faroe Islands (Betz 1981), County Antrim, Northern Ireland (Walker 1951), Italy (Passaglia 1970), Melbourne area, Australia (Vince 1989), Nova Scotia, Canada (Walker and Parsons 1922), and Paterson, New Jersey, United States (Peters and Peters 1978). For all of these there are almost no studies on the conditions of origin of chabazite. However, in eastern Iceland Walker (1960) found regional occurrence of chabazite with thomsonite in the upper most zone of zeolites in amygdules of olivine basalt flows. The boundary with the next lower zone with analcime cuts across flow boundaries, showing that zeolite zones were formed long subsequent to eruption and cooling of the lavas. The temperatures at which similar ones have formed in geothermal areas of Iceland, summarized by Kristmannsdóttir and Tómasson (1978), indicate that chabazite probably forms at temperatures less than 70°C.The thick sections basaltic lava exposed on Disko Island and Nuussuaq Peninsula, central West Greenland, exhibit the effects regional low grade and metamorphism and hydrothermal alteration (Neuhoff et al. 2006). Regional metamorphism of the upper Paleocene lava formation, the Maligât Formation, produced early mixed dioctahedral–trioctahedral smectite followed by chabazite and thomsonite. This same assemblage persists into the upper portions of the underlying Vaigat Formation, where the chabazite–thomsonite assemblage is replaced at depth by an assemblage dominated by mafic phyllosilicates, thomsonite, chabazite, analcime, natrolite, and gonnardite.
Hydrothermal alteration.
Active geothermal systems. Chabazite minerals have not been found in drill core from steam wells in geothermal areas hosted by silicic volcanic rocks, such as Yellowstone National Park, Wyoming, and Wairakei, New Zealand. However, chabazite (species unknown) has been found in the geothermal areas in the basaltic rocks of Iceland. It occurs in the shallowest levels of the low-temperature fields near Reykjavík, Thorlálshöfn, and Akureyi, forming at temperatures below about 70°C. It is rare or unreported from the high-temperature fields, such as Krafla (Kristmannsdóttir and Tómasson 1978).
Late stage, deuteric alteration. Chabazite-Sr occurs in a thin aegerine-K-feldspar pegmatite cutting nepheline and nosean syenite of the Lovozero alkaline massif at Suoluaiv Mountain. It is associated with analcime, gonnardite, and phillipsite, vinogradovite, låvenite, and seidozerite (Pekov et al. 2000). Chabazite-Na also occurs in some miarolitic cavities in pegmatite dikes, for example at Mont Saint-Hilaire, Quebec (Horváth and Gault 1990) and at Ilímaussaq, Greenland (Petersen and Secher 1993).
The chabazite-Mg found in the basalt cavities of the Karikás-tető quarry of Prága Hill near Bazsi, West Hungary, is interpreted to have formed by hydrothermal alteration of feldspar and volcanic glass, in a closed system with Mg-rich solutions (Montagna et al. 2010).
Fractures and cavities in granitic gneiss. Chabazite minerals occur in a few other kinds of hydrothermally altered rocks, such as in the core zone of pegmatite dikes and alteration along fractures in gneiss. Many localities in Switzerland, such as in seams in gneiss and on smoky quartz in alpine-cleft environments at Schattig Wichel, Val Giuv, Tavetsch and Gibelsbach, near Fiesch, Switzerland (Stalder et al. 1973 and Armbruster et al. 1994). |
| |
Armbruster, T., Kohler, T., Meisel, T., Nägler, T., Götzinger, M.A., Stalder, H.A. 1996. The zeolite, fluorite, quartz assemblage of the fissures at Gibelsbach, Fiesch (Valais, Switzerland): crystal chemistry, REE patterns, and genetic speculations. Schweiz. Mineral. Petrogr. Mitt. 76, 131-146.
Betz, V. 1981. Zeolites from Iceland and the Faeroes. Min. Rec., 12, 5-16.
Born, I. von 1772. Lithophylacium Bornianum 1, Prague, pp. 46.
Bosc d’Antic, L. 1792. Mémoire sur la chabazie. J. d’Histoire Naturelle 2, 181-184.
Bowman, R.S. 2003. Applications of surfactant-modified zeolites to environmental remediation. Microporous and Mesoporous Materials 61, 43-56.
Breithaupt, A. 1818.Ergänzungen und Berichtigungen zu dem applikativen Theil. In, Hoffmann, C.A.S. ed., Handbuch der Mineralogie, 4, Abt. 2. Freiberg, p. 41.
Colella, C., de’Gennaro, M., and Aiello, R. 2001. Use of zeolitic tuff in the building industry. In D.L. Bish and D.W. Ming (eds) Natural Zeolites: Occurrence, Properties, Applications. Rev in Mineral. and Geochem. Vol. 45, Washington, D.C., 551-587.
Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillmanns, E., and Vezzalini, G. 1997. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 35, 1571-1606.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004). Rock Forming Minerals. vol. 4B Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites. The Geological Society, London.
de’Gennaro, M., Adabbo, M. and Langella, A. 1995. Hypothesis on the genesis of zeolites in some European volcaniclastic deposits. In Ming, D.W. and Mumpton, F.A. (eds). Natural Zeolites ‘93, Int. Comm. Natural Zeolites, Brockport, New York, 51-67.
de’Gennaro, M., Capelletti, P., Langella, A., Perrotta, A. and Scarpati, C. 2000. Genesis of eolites in the Neapolitan Yellow Tuff: geological, volcanological, and mineralogical evidence. Contrib. Miner. Petrol. 139, 17-35.
de’Gennaro, M. and Franco, E. 1988. Mineralogy of Italian sedimentary phillipsite and chabazite. In Kalló, D. and Sherry, H.S. (eds). Occurrence, Properties and Utilization of Natural Zeolites. Akadémiai Kiadó, Budapest, 87-108.
Dickinson, W.W. and Grapes, R.H. 1997. Authigenic chabazite and implications for weathering in Sirius Groupdiamictite, Table Mountain, dry valleys, Antarctica. Jour. Sed. Res, Sec. A: Sed. Petrol., Proc. 67, 815-820.
Gibson, E.K., Wentworth, S.J. and McKay, D.S. 1983. Chemical weathering and diagenesis of a cold desert soil from Wright Valley, Antarctica: An analog of martian weathering processes. Proc. XIII Lunar Planet. Sic. Conf., Part 2, J. Geophy. Res. 88, A912-A928.
Gottardi, G. and Obradovic, J. 1978. Sedimentary zeolites in Europe. Fortschr. Miner. 56, 316-366.
Gualtieri, A. and Passaglia, E. 2006. Rietveld structure refinement of NH4-exchanged natural chabazite, Eur. J. Mineral. 18, 351-359.
Gude, A. J.,3rd and Sheppard, R.A. 1966. Silica-rich chabazite from the Barstow Formation, San Bernardino County, Southern California. Am. Mineral. 51, 909-915.
Gude, A.J.,3rd and Sheppard, R.A. 1988. A zeolitic tuff in a lacustrine facies of the Gila Conglomerate near Buckhorn, Grant County, New Mexico. U.S. Geol. Surv., Bull. 1763., 22 pp.
Hay, R.L. 1964. Phillipsite of saline lakes and soils. Am. Mineral. 49, 1366-1387.
Hay, R.L. 1970. Silicate reaction in three lithofacies of a semiarid basin, Olduvai Gorge, Tanzania. Miner. Soc. Amer. Spec. Paper 3, 237-255.
Hay, R.L. 1978. Geologic occurrence of zeolites. In Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 135-143.
Hay, R.L. and Sheppard, R.S. 1977. Zeolites in open hydrologic systems. In Mumpton, F.A. (ed). Mineralogy and Geology of Natural Zeolites, Miner. Soc. Am., Short Course Notes, v. 4, 93-102.
Horváth, L. and Gault, R.A. 1990. The mineralogy of Mont Saint-Hilaire, Quebec. Min. Rec. 21, 284-359.
Ishizuka, H. 1985. Prograde metamorphism of the Horokanai Ophiolite in the Kamuikotan Zone, Hokkaido, Japan. J. Petrol., 26, 391-417.
Kristmannsdóttir, H. and Tómasson, J. 1978. Zeolite zones in geothermal areas in Iceland. in Natural Zeolites, Occurrence, Properties, Use, Pergamon Press, Oxford., 277-284.
Langella, A., Cappelletti, P. and De’Gennaro, M. 2001. Zeolites in closed hydrologic systems. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 235-260.
Lenzi, G. and Passaglia, E. 1974. Fenomeni di zeolitizzazione nelle formazioni vulcaniche della regione sabatina. Boll. Soc. Geol. Ital. 93, 623-645.
Liou, J.G. 1979. Zeolite facies metamorphism of basaltic rocks from the East Taiwan Ophiolite. Amer. Mineral. 64, 1-14.
Mazzi, F. and Galli, E. 1983. The tetrahedral framework of chabazite. N. Jb. Miner. Mh., 461-480.
Ming, D.W. and Boettinger, J.L. 2001. Zeolites in Soil Environments. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 323-346.
Montagna, G., Bigi, S., Kónya, P., Szakáll, S. and Vezzalini, G. 2010. Chabazite-Mg: a new natural zeolite of the chabazite series. Amer. Mineral. 95, 939-945.
Neuhoff, P.S., Rogers, K.L., Stannius, L.S., Bird, D.K., and Pederson, A.K. 2006. Regional very low-grade metamorphism of basaltic lavas, Disko--Nuussuaq region, West Greenland. Lithos 92, 33-54.
Papke, K.G. 1972. Erionite and other associated zeolites in Nevada. Nev. Bur. Mines and Geol., Bull. 79, 79 pp.
Passaglia, E. 1970. The crystal chemistry of chabazites. Am. Mineral. 55, 1278-1301.
Passaglia, E. and Vezzalini, G. 1985. Crystal chemistry of diagenetic zeolites in volcanoclastic deposits of Italy. Contrib. Miner. Petrol. 90, 190-198.
Passaglia, E., Vezzalini, G., and Carnevali, R. 1990. Diagenetic chabazites and phillipsites in Italy: crystal chemistry and genesis. Eur. J. Mineral. 2, 827-839.
Pekov, I.V., Turchkova, A.G., Chukanov, N.V., Zadov, A.E. and Grishin, V.G. 2000. Chabazite-Sr, (Sr,Ca)[Al2Si4O12]•6H2O, a new zeolite mineral from Lovozero massif, Kola Peninsula. Proc. Russian Min. Soc. 129, 54-58.
Peters, T.A. and Peters, J.J. 1978. Paterson, New Jersey. Min. Rec. 9, 157-179.
Petersen, O.V. and Secher, K. 1993. The minerals of Greenland. Mineral. Rec. 24, 2, 4- 65.
Romé de l’Isle, J.B.L. 1783. Crystallographie, ou description des formes propres à tour les corps due règne minéral, 2nd edn. Paris, pp. 40.
Sameshima, T. 1978. Zeolites in tuff beds of the Miocene Waitemata Group, Auckland Province, New Zealand. In L.B. Sand and F.A. Mumpton, (eds.) Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 309-317.
Sand, L.B. and Regis, A.J. 1966. An unusual zeolite assemblage, Bowie, Arizona. Geol. Soc. Am., Sp. Paper 87, 145-146.
Sersale, R. 1978. Occurrences and uses of zeolites in Italy. In, Sand, L.B. and Mumpton, F.A. (eds). Natural Zeolites: Occurrence, Properties, Use, Pergamon Press, Elmsford, New York, 285-302.
Sheppard, R.A. 1994. Zeolitic diagenesis of tuffs in Miocene lacustrine rocks near Harney Lake, Harney County, Oregon. U.S. Geol. Surv., Bull. 2108, 28 pp.
Sheppard, R.A. and Gude, A.J. 3rd. 1969. Diagenesis of tuffs in the Barstow Formation, Mud Hills San Bernardino County, California. U.S. Geol. Surv., Prof. Paper 634, 35 pp.
Sheppard, R.S. and Gude, A.J.3rd. 1970. Calcic siliceous chabazite from the John Day Formation, Grant County Oregon. U.S. Geol. Surv. Prof. Pap. 700-D, 176-180.
Sheppard, R.A. and Gude, A.J. 3rd. 1973. Zeolites and associated authigenic silicate minerals in tuffaceous rocks of the Big Sandy Formation, Mohave County, Arizona. U.S. Geol. Surv., Prof. Paper 830, 36 pp.
Sheppard, R.S. and Hay, R.L. 2001. Formation of zeolites in open hydrologic systems. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 261-276.
Smith, J.V. 1988. Topochemistry of zeolites and related materials. 1. Topology and chemistry. Chem. Rev. 88, 149-182.
Stalder, H.A., de Quervain, F., Niggli, E., Graeser, St. and Jenny, V. 1973. Die Mineralfunde der Schweiz, revised edition of Parker, R.L. Die Mineralfunde der Schweizer Alpen, Wepf and Co, Verlag, Basel. 433 pp.
Surdam, R.C. and Eugster, H.P. 1976. Mineral reactions in the sedimentary deposits of the Lake Magadi region, Kenya. Geol. Soc. Am. Bull. 87, 1739-1752.
Vince, D. 1989. Melbourne., in Birch, W.D. Zeolites of Victoria, Mineral. Soc. Victoria (Australia), Sp. Pub. No. 2, 1-30.
Vitali, F., Blanc, G., and Larqué,P. 1995. Zeolite distribution in volcaniclastic deep-sea sediments from the Tonga Trench margin (SW Pacific). Clays and Clay Minerals 43, 92-104.
Walker, J.P.L. 1951. The amygdale minerals in the Tertiary lavas of Ireland. I. The distribution of chabazite habits and zeolites in the Garron plateau area, County Antrim. Min. Mag. 29, 773-791.
Walker, J.P.L. 1960. Zeolite zones and dike distribution in relation to the structure of the basalts of eastern Iceland. J. Geol., 68, 515-528.
Walker, T.L. and Parsons, A.L. 1922. The zeolites of Nova Scotia. University of Toronto Studies, Geological Sciences, no. 14, 13-73. |