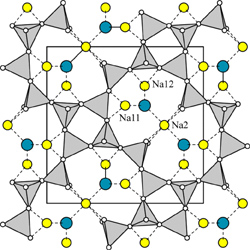
| |
Ataman, G. and Beseme, P.
(1972) Découverte de l’analcime sédimentaire en Anatolie du
nord-ouest (Turquie): minéralogie, genèse, paragènese. Chem.
Geol. 9, 203-225.
Ataman, G. and Gündogdu, N. (1982) Analcimic zones in the
Tertiary of Anatolia and their geologic positions. Sediment.
Geol. 31, 89-99.
Baldwin, J.L. (1944) Tupungate oil field, Mendoza, Argentina. Amer.
Assoc. Petrol. Geol. Bull. 28, 1455-1484.
Bargar, K.E. and Beeson, M.H. (1985) Hydrothermal alteration in
Research Drill Hole Y-3, Lower Geyser Basin, Yellowstone National
Park, Wyoming. U.S. Geol. Surv., Prof. Paper 1054-C,
23 pp.
Boles, J.R. and Coombs, D.S. (1975) Mineral reactions in zeolitic
Triassic tuff, Hokonui Hills, New Zealand. Geol. Soc. Amer.,
Bull. 86, 163-173.
Broxton, D.E., Bish, D.L., and Warren, R.G. (1987) Distribution
and chemistry of diagenetic minerals at Yucca Mountain, Nye
County, Nevada. Clays and Clay Miner. 35, 89-110.
Colella, C., de' Gennaro, M,. and Aiello, R. (2001) Use of
zeolitic tuff in the building industry. In: Bish, D.L.
and Ming, D.W. (eds.) Natural Zeolites: Mineralogy,
Occurrence, Properties, Applications, Reviews in Mineralogy
& Geochemistry, Mineralogical Society of America, Vol. 45,
Washington, D.C., 551-587.
Colella, C. (2007) Natural zeolites and environment. In: ?ejka,
J., van Bekkum, H., Corma, A. and Schueth, F. (eds.)
Introduction to Zeolite Science and Practice. 3rd Revised
Edition, Studies in Surface Science and Catalysis No. 168,
Elsevier, Amsterdam, 999-1035.
Coombs, D.S. (1954) The nature and alteration of some Triassic
sediments from Southland, New Zealand. Trans. Roy. Soc. N.Z.
82, 65-109.
Coombs, D.S. (1955) X-ray observations of wairakite and non-cubic
analcime. Mineral. Mag. 30, 699-708.
Coombs, D.S. and Whetten, J.T. (1967) Composition of analcime
from sedimentary and metamorphic rocks. Geol. Soc. Amer.,
Bull. 78, 269-282.
Cruciani, G. and Gualtieri, A. (1999) Dehydration dynamics of
analcime by in situ synchrotron powder diffraction. Am.
Mineral. 84, 112 €“119.
Cundari, A. and Graziani, G. (1964) Prodotti di alterazione della
leucite nelle vulcaniti vicane. Period. Miner. 33,
35-52.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004) Rock
Forming Minerals. vol. 4B. Framework Silicates: Silica Minerals,
Feldspathoids and the Zeolites. The Geological Society,
London.
DeMark, R.A. (1984) Minerals of Point of Rocks, New Mexico. Mineral.
Rec. 15, 149-156.
Echle, W. (1975) Zusammensetung und Entstehung sedimentärer
Analcime in Jungtertiären pyroklastischen Gestein nördlich
Mihaliççik, Westanatolien, Türkei. Neues Jahrb. Miner. Abh.
124, 128-146.
Fyfe, W.S., Turner, F.J., and Verhoogen, J. (1958) Metamorphic
reactions and metamorphic facies. Geol. Soc. Am. Mem.
73, 259 pp.
Gatta, G.D., Nestola, F. and Boffa Ballaran, T. (2006) Elastic
behavior, phase transition, and pressure induced structural
evolution of analcime. Am. Mineral. 91, 568 - 578.
Gall, Q. and Hyde, R. (1989) Analcime in lake and lake-margin
sediments of the Carboniferous Rocky Brook Formation, western
Newfoundland, Canada. Sedimentology 36, 875-887.
Goble, R.J., Treves, S.B., and Ghazi, A.M. (1993) Comparison of
the Rainy ridge analcime phonolite sill and the Crowsnest
volcanics, Alberta, Canada. Can. J. Earth Sci. 30,
1644-1649.
Gottardi, G. and Galli, E. (1985) Natural Zeolites.
Springer-Verlag, Berlin.
Gottardi, G. and Obradovic, J. (1978) Sedimentary zeolites in
Europe. Fortschr. Mineral. 56, 316-366.
Gündogdu, M.N., Yalcin, H., Temel, A., and Clauer, N. (1996)
Geological, mineralogical and geochemical characteristics of
zeolite deposits associated with borates in the Bigadic, Emet and
Kirka Neogene lacustrine basins, western Turkey. Mineral.
Deposita 31, 492-513.
Gupta, A.K. and Fyfe, W.S. (1975) Leucite survival: The
alteration to analcime. Can. Mineral. 13, 361-363.
Harada, K., Tanaka, K., and Nagashima, K. (1972) New data on the
analcime-wairakite series. Am. Mineral. 57, 924-931.
Harker, A. (1954) Petrology for students. Cambridge
University Press, Cambridge, England. 283 p.
Haüy, R.-J. (1797) Analcime. J. des Mines 5, 278-279.
Haüy, R.-J. (1801) Traité de minéralogie 3. Chez
Louis, Paris, France.
Hay, R.L. (1966) Zeolites and zeolitic reaction in sedimentary
rocks. Geol. Soc. Amer., Spec. Pap. 85, 130 pp.
Hay, R.L. (1970) Silicate reactions in three lithofacies of a
semi-arid basin, Olduvai Gorge, Tanzania. Min. Soc. Amer.,
Spec. Pap. 3, 237-255.
Hay, R.L. and Sheppard, R.S. (1977) Zeolites in open hydrologic
systems. In: Mumpton, F.A. (ed.) Mineralogy and
Geology of Natural Zeolites, Miner. Soc. Am., Short Course
Notes, v. 4, 93-102.
Hazen, R.M. and Finger, L.W. (1979) Polyhedral tilting: A common
type of pure displacive phase transition and its relationship to
analcite at high pressure. Phase Trans. 1, 1-22.
Henderson, C.M.B. and Gibb, F.G.F. (1983) Felsic mineral
crystallization trends in differentiating alkaline basic magmas. Contr.
Min. Petr.84, 355-364.
Hey, M.H. (1930) Studies on the zeolites. I. General review. Mineral.
Mag. 22, 422-437.
High, L.R. and Picard, M.D. (1965) Sedimentary petrology and
origin of analcime-rich Pogo Agie Member, Chugwater Formation
(Triassic), west-central Wyoming. J. Sed. Petrol. 35,
49-70.
Horvath, L. and Gault, R.A. (1990) The mineralogy of Mont
Saint-Hilaire, Quebec. Mineral. Rec. 21, 284-359.
Iijima, A. (1978) Geological occurrences of zeolite in marine
environments. In: Sand, L.B. and Mumpton, F.A. (eds.) Natural
Zeolites: Occurrence, Properties, Use, Pergamon Press,
Elmsford, New York, 175-198.
Joulia, F., Bonifas, M., Camez, T., Millot, G., and Weid. R.
(1959) Découverte d’un important niveau d’analcimolite
gresèuse dans le Continental intercalaire de l’ouest de l’Air
(Sahara central). . Dakar Service de Géologie et de
Prospection Minière, 40 pp.
Kastner, M. and Stonecipher, S.A. (1978) Zeolites in pelagic
sediments of the Atlantic, Pacific, and Indian Oceans. In:
Sand, L.B. and Mumpton, F.A. (eds.) Natural Zeolites:
Occurrence, Properties, Use, Pergamon Press, Elmsford, New
York, 199-220.
Keith, T.E.C., Thompson, J.M., and Mays, R.E. (1983) Selective
concentration of cesium in analcime during hydrothermal
alteration, Yellowstone National Park, Wyoming. Geochim.
Cosmochim. Acta 47,795-804.
Keller, W.D. (1952) Analcime in the Popo Agie Member of the
Chugwater Formation. J. Sed. Petrol. 22, 70-82.
Keller, W.D. (1953) Analcime in the Chinle formation of Utah
correlative with the Pop Agie of Wyoming. J. Sed. Petrol.
23, 10-12.
Koshiya S., Okami, K., Hayasaka, Y., Uzawa, M., Kikuchi, Y., and
Doi, N. (1994) On the hydrothermal mineral veins developed in the
Takinoue geothermal area, northeast Honshu, Japan. J.
Geotherm. Res. Soc. Japan 6, 1-24.
Luhr, J.F. and Giannetti, B. (1987) The Brown Leucitic Tuff of
Roccamonfina Volcano (Roman Region, Italy). Cont. Mineral.
Petrol. 95, 420-436.
Mazzi, F. and Galli, E. (1978) Is each analcime different?
Am. Mineral. 6, 448-460.
Murray, J. and Renard, A.F. (1891) Report on Scientific
Results of the Voyage of "H.M.S. Challenger" during the years
1873-1876; Deep Sea Deposits. Johnson Reprint Co., London,
525 pp.
Obradovic, J. (1988) Occurrences and genesis of sedimentary
zeolites in Serbia. In: Kalló, D. and Sherry, H.S.
(eds.) Occurrence, Properties and Utilization of Natural
Zeolites. Akadémiai Kiadó, Budapest, 59-70.
Obradovic, J., Dimitrijevic, R., Visic, N., and Kasanin, M.
(1995) Analcime from Teriary lacustrine basins in Serbia. Annales
géol. de la péninsula balkanique 59, 255-273 (MA 97M/0839).
Otálora, G. (1964) Zeolites and related minerals in Cretaceous
rocks of east-central Puerto Rico. Am. J. Sci. 262,
726-734.
Pearce, T.H. (1970) The analcite-bearing volcanic rocks of the
Crowsnest Formation, Alberta. Can. J. Earth Sci., 7,
46-66.
Pearce, T.H. (1993) Analcime phenocrysts in igneous rocks:
Primary or secondary?---Discussion. Am. Mineral. 78,
225-229.
Pekov, I.V. (2000). Lovozero Massif: History, Pegmatites,
Minerals. Ocean Pictures Ltd., Moscow, Russia. 484 pp.
Polikarpov, A.I., Polyakovskiy, V. Ya., and Kiseleva, O. V.
(1986) Analcime and associated argillaceous minerals in
supersaline deposits of the Solikamsk Basin. Zap. Vses.
Mineral. Ob. 115, 86-93.
Raade, G., Åmli, R., Mladeck, M.H., Din, V.K., Larsen, A.O., and
Åsheim, A. (1983) Chiavennite from syenite pegmatites in the Oslo
Region, Norway. Am. Mineral. 68, 628-633.
Remy, R.R. and Ferrell, R.E. (1989) Distribution and origin of
analcime in marginal lacustrine mudstones of the Green River
Formation, south-central Uintsa Basin, Utah. Clays and Clay
Miner. 37, 419-432.
Renaut, R.W. (1993) Zeolitic diagenesis of late Quarternary
fluviolacustrine sediments and associated calcrete formation in
the Lake Bogoria Basin, Kenya Rift Valley. Sedimentology,
40, 271-301.
Ross, C.S. (1928) Sedimentary analcite. Am. Mineral.
13, 195-197.
Roux, J. and Hamilton, D.L. (1976) Primary igneous analcite--an
experimental study. J. Petrol. 17, 244-257.
Saha, P. (1959) Geochemical and X-ray investigation of natural
and synthetic analcites. Am. Mineral. 44, 300-313.
Seki, Y. (1969) Facies series in low-grade metamorphism. J.
Geol. Soc. Japan 75, 255-266.
Sheppard, R.A. and Gude, A.J. 3rd. (1969) Diagenesis of tuffs in
the Barstow Formation, Mud Hills San Bernardino County,
California. U.S. Geol. Surv., Prof. Paper 634, 35 pp.
Sheppard, R.A. and Gude, A.J. 3rd. (1973) Zeolites and associated
authigenic silicate minerals in tuffaceous rocks of the Big Sandy
formation, Mohave County, Arizona. U.S. Geol. Surv., Prof.
Paper 830, 36 pp.
Sheppard, R.A. and Hay, R.L. (2001) Formation of zeolites in open
hydrologic systems. In: Bish, D.L. and Ming, D.W. (eds.)
Natural Zeolites: Occurrence, Properties, Applications,
Reviews in Mineralogy & Geochemistry, Mineralogical Society of
America, Vol. 45, Washington, D.C., 261-276.
Surdam, R.C. (1966) Analcime-wairakite mineral series. Geol.
Soc. Am. Spec. Paper 87, 169-170.
Tang, Z., Parnell, J., and Longstaffe, F.J. (1997) Diagenesis of
analcime-bearing reservoir sandstones: the Upper Permian
Pingdiquan Formation, Junggar, Basin, northwest China. J.
Sediment. Res. 67, 486-498.
Taylor, W.H. (1930) The crystal structure of analcite (NaAlSi2O6
€¢H2O). Z. Kristallogr. 74, 1-19.
Teruggi, M.E. (1964) A new and important occurrence of
sedimentary analcime. J. Sed. Petrol. 34, 761-767.
Teruggi, M.E. and Andreis, R.R. (1963) Revision de las zeiktas
con especial referencia a su importancia sedimentologia. Revista
de la Asoc. Geol. Argentina 18, 73-95.
Tibljas, D. and Kovacic, M. (2018). Sedimentary Zeolite Deposits
in Croatia. International Zeolite Association, Commission on
Natural Zeolites, Catalog of Deposits: Croatia.
http://www.iza-online.org/natural/default.htm
Utada, M. (1988) Occurrence and genesis of hydrothermal zeolites
and related minerals from the Kuroko-type mineralization areas,
Japan. In: Kalló, D. and Sherry, H.S. (eds.) Occurrence,
Properties and Utilization of Natural Zeolites. Akadémiai
Kiadó, Budapest, 39-48.
van de Kamp, P.C. and Leake, B.E. (1996) Petrology, geochemistry,
and Na metasomatism of Triassic-Jurassic non-marine clastic
sediments in the Newark, Hartford, and Deerfield rift basins,
northeastern USA. Chem. Geol. 133, 89-124.
Vanderstappen, R. and Verbeek, T. (1959) Présence d’analcime
d’origine sédimentaire dans le Mésozoique du Congo. Belge
Géol., Paleont., et Hydrol. Bull. 88, 417-421.
Vanderstappen, R. and Verbeek, T. (1964) Analcime et mineraux
argileux des formations geologiques de la cuvette congolaise
(Republique du Congo). Musee Royal de l'Afrique Centrale,
Belgique Annales, Serie in-8 °, Sciences Géologiques, no.,
88 pp.
Van Houten, F.B. (1960) Composition of Upper Triassic Lockatong
argillite, west-central New Jersey. Jour. Geol. 68,
666-669.
Van Houten, F.B. (1962) Cyclic sedimentation and the origin of
analcime-rich Upper Triassic Lockatong Formation, west-central New
Jersey and adjacent Pennsylvania. Am. Jour. Sci. 260,
561-576.
Van Houten, F.B. (1965) Composition of Triassic Lockatong and
associated formations of Newark Group, central New Jersey and
adjacent Pennsylvania. Am. Jour. Sci. 263, 825-863.
Whateley, M.K.G., Querol, X., Fernández-Turiel,
J.L., and Tuncali, E. (1996) Zeolites in Teriary coal from the
Cayirhan mine, Beypazari, Turkey. Mineral. Deposita 31,
529-538.
Wilkinson, J.F.G. (1965) Some feldspars, nephelines and analcimes
from the Square Top Intrusion, Nundle, N.S.W. J. Petrol.
6, 420-444.
Wilkinson, J.F.G. (1968) Analcimes from some potassic igneous
rocks and aspects of analcime-rich igneous assemblages. Contr.
Min. Petr. 18, 252-269.
Zhitova E. S., Popov M. P. and Zolotarev A. A. jr. (2017)
Analcime of the Mariinskoe deposit (Urals emerald mines, the
Middle Urals): chemical composition and crystal structure. Zapiski
RMO 146, 111-120.
Updated: April 2025.
|