Standard Reaction for Acidity Characterization:
Ethylbenzene Disproportionation
July 31, 2002
INTRODUCTION
The Catalysis Commission is one of the five working groups of the International Zeolite Association (IZA) that intend to serve the zeolite community by providing uniform nomenclature and reliable synthesis and reaction procedures. For industrial or academic groups that are not familiar with zeolite catalytic reactions, or even for established teams, it is desirable to compare the results obtained in their own, often home-built, reactors with data obtained by other groups. By publishing established procedures and results of test reactions, it is the intention of the Catalysis Commission to provide readily accessible reference data. The present paper reports on the first of such reference reactions, namely the disproportionation of ethylbenzene over LaNaY zeolite. In the past similar initiatives have been undertaken by consortia of European catalysis laboratories, for instance, with the standard hydrogenation catalyst EuroPt-1 or the hydroxylation catalyst EuroTS-1.
The tests have been carried out in the following five laboratories:
| Dirk E. De Vos | Center for Surface Chemistry and Catalysis K.U. Leuven Kasteelpark Arenberg 23 B-3001 Leuven Belgium |
| Stefan Ernst | Fachbereich Chemie Universität Kaisersläutern P.O. Box 3049 D-67653 Kaisersläutern Germany |
| Carlo Perego | Enitecnologie SpA Catalysis Department Via Maritano 26 I-20097 San Donato Milanese Italy |
| Cyril O'Connor | Faculty of Engineering University of Cape Town Rondebosch 7700 South Africa |
| Michael Stöcker | SINTEF Applied Chemistry Department of Hydrocarbon Process Chemistry P.O. Box 124 Blindern N-0314 Oslo Norway |
Apart from the detailed standard procedure for catalyst preparation and catalytic reaction, we also give a format for data calculation and presentation. In the data presentation, the five laboratories involved have been labeled in a random way as L1, L2, L3, L4 and L5. We briefly indicate how our standardized results fit within previously reported literature data. Finally the problems and pitfalls that can be encountered in obtaining reproducible results will be discussed.
EXPERIMENTAL
Reaction Scheme

Reactor Scheme
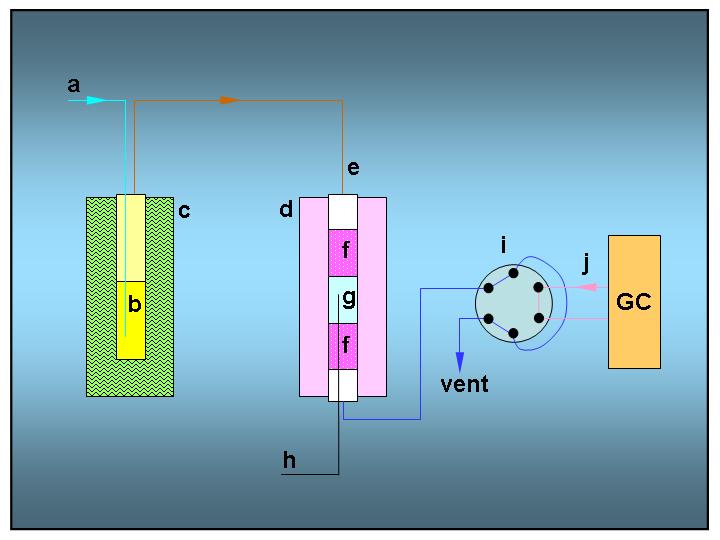
| Reactor setup for ethylbenzene disproportionation: | ||
| (a) | N2 gas flow | |
| (b) | saturator with ethylbenzene | |
| (c) | thermostated bath | |
| (d) | tube furnace for quartz reactor | |
| (e) | quartz reactor | |
| (f) | inert packing material (quartz) | |
| (g) | catalyst bed | |
| (h) | thermocouple | |
| (i) | 6-way sampling valve for on-line GC analysis | |
| (j) | GC carrier gas (He or H2) | |
Preparation of LaNaY Catalyst
LaNaY is prepared starting from NaY zeolite (Si/Al = 2.4) from Degussa.
The zeolite is first saturated with water vapor in a desiccator till to a water content of 24.2 wt.% (TGA).
166.5 g of the moist zeolite (corresponding to 126.2 g dry zeolite) is suspended in 400 ml water and then 41.0 g La(NO3)3•6H2O in 50 ml water is added.
The pH is adjusted with dilute NaOH until it is above 5.5.
After adjusting the volume to 500 ml, the suspension is heated at 353 K under stirring for 18 h.
The suspension is filtered while hot and washed with 1.5 l water.
After re-suspension of the filter cake in 400 ml water, a second exchange is performed with an identical procedure, followed by a third and a fourth exchange.
After the 4th exchange, the zeolite is washed until nitrate free, air-dried, and dried for 24 h in an oven at 373 K.
Test Procedure
Ethylbenzene purification: impurities such as ethylbenzene hydroperoxide or 1-phenylethanol are removed by passing the ethylbenzene feed through a column which contains alumina freshly activated by calcination at 723 K. Pure ethylbenzene should not give any color when 1 ml is mixed with about 0.2 ml of concentrated sulfuric acid.
0.29 g dry catalyst is diluted with 3 ml quartz and charged into the reactor.
Pretreatment: the catalyst is heated in a N2 flow (50 ml/min) at a heating rate of 2 K/min up to 523 K and then held at 523 K for 12 h.
The reaction is stated after 2 hours of stabilization at the reaction temperature
Reaction conditions:
N2 flow rate in ethylbenzene saturator: 40 ml/min, i.e. 1 mmol/h for ethylbenzene
Reaction temperature: 453, 523 and 593 K.
On-line GC analysis with CP-Sil5 column.
Calculation of Conversion and Yields
The corrected GC peak area ai, mole-proportional figure, for each compound i is calculated by multiplying Ai (GC peak area) with an FID response factor (fi) and dividing by the molecular weight Mi:
ai = Ai • fi / Mi
Typical FID response factors are: fBz = 1.000, fE-Bz = 1.019, fDE-Bz = 1.031.
Conversion (X) and yields (Y) are calculated as follows:
XE-Bz = (aBz + aDE-Bz)/(aBz + aE-Bz + aDE-Bz)
YBz = 2•aBz/(aBz + aE-Bz + aDE-Bz)
YDE-Bz =2•aDE-Bz/(aBz + aE-Bz + aDE-Bz)
CATALYTIC RESULTS
The experimental results are presented in Figures 1-3 and Table 1. Details will be discussed in the full paper to be published in Microporous and Mesoporous Materials soon. Their highlights are outlined below:
Ethylbenzene conversion increases during an induction period; it then stays stable, or particularly at the higher temperatures it slowly decreases due to catalyst deactivation.
In the initial phase, much more benzene is formed than diethylbenzenes. Therefore, the ratio of the molar yields YDE-Bz/YBz increases during the induction to eventually reach a constant value.
The ratio of the molar yields YDE-Bz/YBz markedly decreases with increasing reaction temperature.
Early in the induction period, para-diethylbenzene may be the dominant isomer, but the selectivity quickly decreases at the expense of meta- and ortho-diethylbenzene.
The data scattering is usually due to the experimental deviation in each individual lab. The best agreement among the different labs is found in the distribution of diethylbenzene isomers.
Fig. 1: Conversion of ethylbenzene (XE-Bz)
as a function of time on stream at W/FE-Bz= 290 g•h/mol and
453 K: L1 (![]() ), L2 (■),
L3 (▲), L4 (x) and L5 (*).
), L2 (■),
L3 (▲), L4 (x) and L5 (*).
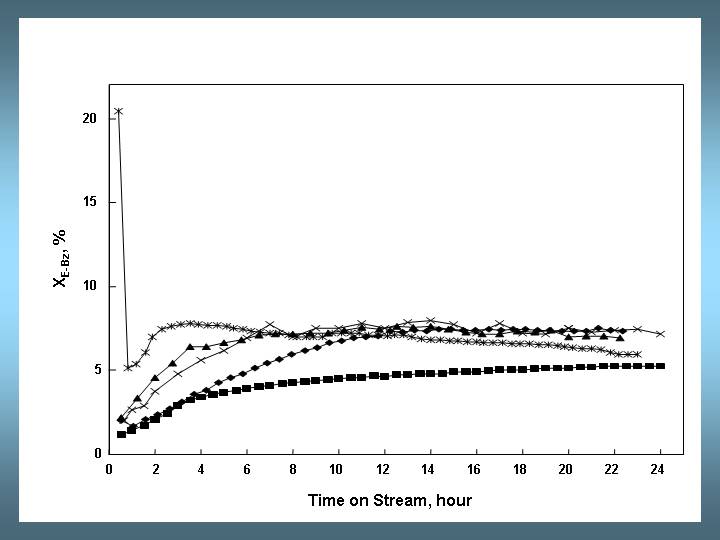
Fig. 2: Molar Ratio of diethylbenzene yield (YDE-Bz) over benzene yield (YBz) as a function of time on stream. Conditions as in Fig. 1 but 523 K.
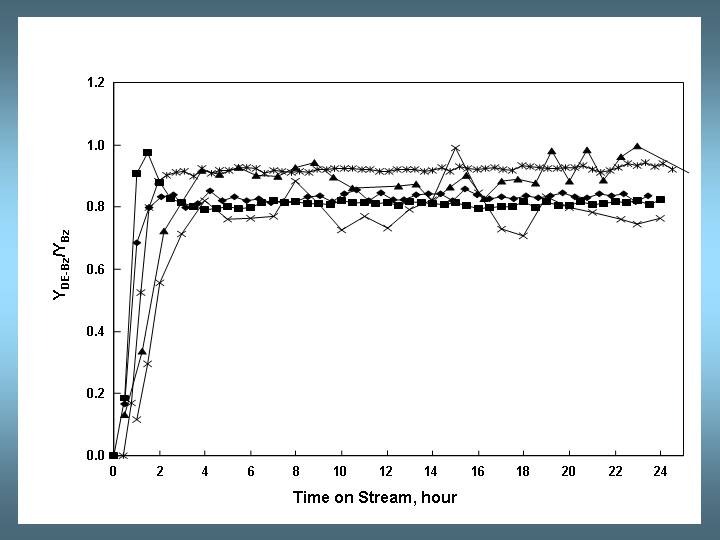
Fig. 3: Selectivities to diethylbenzene isomers as a function of time on stream. Conditions as in Fig. 1.
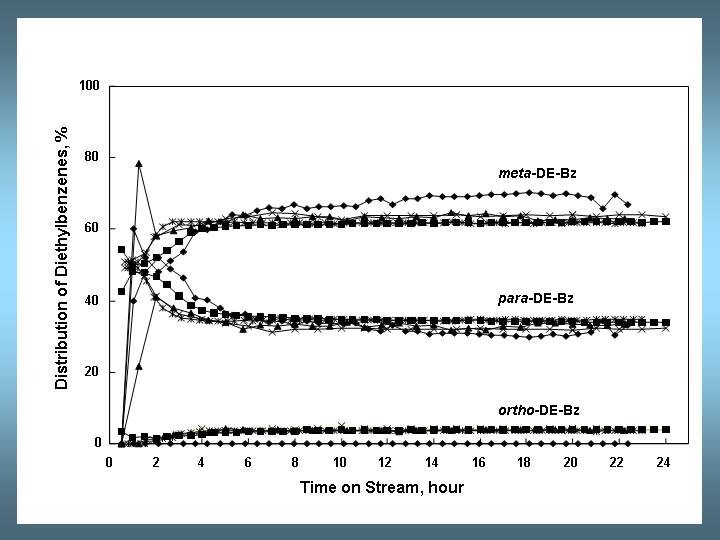
Table 1: Statistical evaluation of reaction parameters after 20 h of time on stream
|
|
453 K |
523 K |
593 K |
|
XE-Bz, % |
6.7 ± 1.0 |
13.7 ± 3.2 |
13.9 ± 4.2 |
|
YBz, % |
7.0 ± 1.3 |
20.9 ± 3.6 |
16.8 ± 5.9 |
|
YDE-Bz, % |
6.4 ± 0.7 |
16.6 ± 2.8 |
11.0 ± 2.9 |
|
YBz/YDE-Bz |
0.92 ± 0.11 |
0.85 ± 0.06 |
0.67 ± 0.13 |
DISCUSSION
According to Weitkamp and Karge groups [1], the induction period observed for 12-MR zeolites corresponds to the formation of higher alkylated aromatics such as polyalkylated diphenylethanes. Since polyalkylated diphenylethanes contain more than one ethyl group per aromatic nucleus, the molar yield of benzene YBz is initially higher than that of diethylbenzenes YDE-Bz (see Fig.2)
At 453 K in the stationary regime the molar yields of benzene YBz and diethylbenzenes YDE-Bz are approximately equal (i.e. stoichiometric ethylbenzene disproportionation).
At higher temperatures (523 and 593 K) the molar ratio YDE-Bz /YBz is below 1, as part of ethylbenzene is cracked into benzene and ethylene. Besides, a slowly deactivation is detected probably related to the cracking reaction.
Even in the stationary phase the distribution of diethylbenzene isomers often deviates from the thermodynamic equilibrium. Literature data show that for reactions at 453-473 K, the stationary para selectivity increases in the order H-L < H-Beta ≈ LaNaY < LaNaEMT < H-MCM-22 [2]. The data of the five labs are in excellent agreement with previously reported data on LaNaY catalysts. Initially, the para product prevails. In an electrophilic substitution, both ortho and para positions are electronically activated. However, the para position is favored because of steric hindrance of the ethyl group. The meta isomer is thermodynamically more stable and is obtained by secondary isomerization. As the activity of the catalyst increases during the induction period, secondary isomerization leads to more meta isomer via a transalkylation mechanism.
CRITICAL FACTORS
While all observations are qualitatively the same for all labs, the quantitative agreement could have been better. During the work, several critical factors were identified that lead to the following precautions:
Granulated catalysts should be diluted with quartz chips so to avoid a faster deactivation.
Oxygenated aromatics in the impure feed is a further cause of deactivation. Therefore, a highly purified feed is advisable.
The saturator design is crucial: the residence time of the gas flow must be sufficient to ensure the complete saturation of the flow by the vapor of ethylbenzene feed.
Reactor temperature control within 1 K seems critical. High initial conversions may be due to a temperature overshoot. Ventilation of the oven may help to achieve a homogeneous temperature profile.
A delay of 2 hours between pretreatment and reaction start up is advisable.
Quantitative determination of product distributions is largely related to a correct GC analysis procedure: the response factors must be carefully controlled as they can vary with a few percent for different FID detectors.
REFERENCES
1. U. Weiss et al., Stud.
Surf. Sci. Catal., 105 (1997)
973.
2. H.G. Karge et al., Stud. Surf.
Sci. Catal., 84 (1994)
1805.